Redesigning an FKBP-ligand interface to generate chemical dimerizers with novel specificity
- PMID: 9724721
- PMCID: PMC27912
- DOI: 10.1073/pnas.95.18.10437
Redesigning an FKBP-ligand interface to generate chemical dimerizers with novel specificity
Abstract
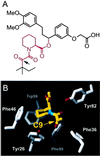
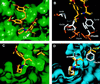
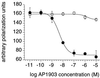
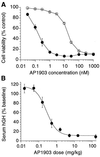
FKBP ligand homodimers can be used to activate signaling events inside cells and animals that have been engineered to express fusions between appropriate signaling domains and FKBP. However, use of these dimerizers in vivo is potentially limited by ligand binding to endogenous FKBP. We have designed ligands that bind specifically to a mutated FKBP over the wild-type protein by remodeling an FKBP-ligand interface to introduce a specificity binding pocket. A compound bearing an ethyl substituent in place of a carbonyl group exhibited sub-nanomolar affinity and 1,000-fold selectivity for a mutant FKBP with a compensating truncation of a phenylalanine residue. Structural and functional analysis of the new pocket showed that recognition is surprisingly relaxed, with the modified ligand only partially filling the engineered cavity. We incorporated the specificity pocket into a fusion protein containing FKBP and the intracellular domain of the Fas receptor. Cells expressing this modified chimeric protein potently underwent apoptosis in response to AP1903, a homodimer of the modified ligand, both in culture and when implanted into mice. Remodeled dimerizers such as AP1903 are ideal reagents for controlling the activities of cells that have been modified by gene therapy procedures, without interference from endogenous FKBP.
Figures
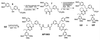
References
-
- Schreiber S L. Science. 1991;251:283–287. - PubMed
-
- Holt D A, Luengo J I, Yamashita D S, Oh H-J, Konialian A K, Yen H-K, Rozamus L W, Brandt M, Bossard M J, Levy M A, et al. J Am Chem Soc. 1993;115:9925–9938.
-
- Hauske J R, Dorff P, Julin S, DiBrino J, Spencer R, Williams R. J Med Chem. 1992;35:4284–4296. - PubMed
-
- Armistead D M, Badia M C, Deininger D D, Duffy J P, Saunders J O, Ting R D, Thomson J A, DeCenzo M T, Futer O, Livingston D J, et al. Acta Crystallogr D. 1995;51:522–528. - PubMed
-
- Babine R E, Bender S L. Chem Rev. 1997;97:1359–1472. - PubMed
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

