Cloning and characterization of a third human lysyl hydroxylase isoform
- PMID: 9724729
- PMCID: PMC27920
- DOI: 10.1073/pnas.95.18.10482
Cloning and characterization of a third human lysyl hydroxylase isoform
Abstract
Lysyl hydroxylase (EC 1.14.11.4), a homodimer, catalyzes the formation of hydroxylysine in collagens. Recently, an isoenzyme termed lysyl hydroxylase 2 has been cloned from human sources [M. Valtavaara, H. Papponen, A.-M. Pirttilä, K. Hiltunen, H. Helander and R. Myllylä (1997) J. Biol. Chem. 272, 6831-6834]. We report here on the cloning of a third human lysyl hydroxylase isoenzyme, termed lysyl hydroxylase 3. The cDNA clones encode a 738 amino acid polypeptide, including a signal peptide of 24 residues. The overall amino acid sequence identity between the processed human lysyl hydroxylase 3 and 1 polypeptides is 59%, and that between the processed lysyl hydroxylase 3 and 2 polypeptides is 57%, whereas the identity to the processed Caenorhabditis elegans polypeptide is only 45%. All four recently identified critical residues at the catalytic site, two histidines, one aspartate, and one arginine, are conserved in all these polypeptides. The mRNA for lysyl hydroxylase 3 was found to be expressed in a variety of tissues, but distinct differences appear to exist in the expression patterns of the three isoenzyme mRNAs. Recombinant lysyl hydroxylase 3 expressed in insect cells by means of a baculovirus vector was found to be more soluble than lysyl hydroxylase 1 expressed in the same cell type. No differences in catalytic properties were found between the recombinant lysyl hydroxylase 3 and 1 isoenzymes. Deficiency in lysyl hydroxylase 1 activity is known to cause the type VI variant of the Ehlers-Danlos syndrome, and it is therefore possible that deficiency in lysyl hydroxylase 3 activity may lead to some other variant of this syndrome or to some other heritable connective tissue disorder.
Figures

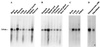

References
-
- Kivirikko K I, Pihlajaniemi T. Adv Enzymol Relat Areas Mol Biol. 1998;72:325–398. - PubMed
-
- Hautala T, Byers M G, Eddy R L, Shows T B, Kivirikko K I, Myllylä R. Genomics. 1992;13:62–69. - PubMed
-
- Yeowell H N, Ha V, Walker L C, Murad S, Pinnell S R. J Invest Dermatol. 1992;99:864–869. - PubMed
-
- Armstrong L C, Last J A. Biochim Biophys Acta. 1995;1264:93–102. - PubMed
-
- Myllylä R, Pihlajaniemi T, Pajunen L, Turpeenniemi-Hujanen T, Kivirikko K I. J Biol Chem. 1991;266:2805–2810. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

