The adenomatous polyposis coli-binding protein EB1 is associated with cytoplasmic and spindle microtubules
- PMID: 9724749
- PMCID: PMC27940
- DOI: 10.1073/pnas.95.18.10596
The adenomatous polyposis coli-binding protein EB1 is associated with cytoplasmic and spindle microtubules
Abstract
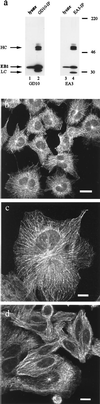
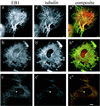
The evolutionarily conserved protein EB1 originally was identified by its physical association with the carboxyl-terminal portion of the adenomatous polyposis coli (APC) tumor suppressor protein, an APC domain commonly mutated in familial and sporadic forms of colorectal neoplasia. The subcellular localization of EB1 in epithelial cells was studied by using immunofluorescence and biochemical techniques. EB1 colocalized both to cytoplasmic microtubules in interphase cells and to spindle microtubules during mitosis, with pronounced centrosome staining. The cytoskeletal array detected by anti-EB1 antibody was abolished by incubation of the cells with nocodazole, an agent that disrupts microtubules; upon drug removal, EB1 localized to the microtubule-organizing center. Immunofluorescence analysis of SW480, a colon cancer cell line that expresses only carboxyl-terminal-deleted APC unable to interact with EB1, demonstrated that EB1 remained localized to the microtubule cytoskeleton, suggesting that this pattern of subcellular distribution is not mediated by its interaction with APC. In vitro cosedimentation with taxol-stabilized microtubules demonstrated that a significant fraction of EB1 associated with microtubules. Recent studies of the yeast EB1 homologues Mal3 and Bim1p have demonstrated that both proteins localize to microtubules and are important in vivo for microtubule function. Our results demonstrate that EB1 is a novel component of the microtubule cytoskeleton in mammalian cells. Associating with the mitotic apparatus, EB1 may play a physiologic role connecting APC to cellular division, coordinating the control of normal growth and differentiation processes in the colonic epithelium.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

