MLL, a mammalian trithorax-group gene, functions as a transcriptional maintenance factor in morphogenesis
- PMID: 9724755
- PMCID: PMC27946
- DOI: 10.1073/pnas.95.18.10632
MLL, a mammalian trithorax-group gene, functions as a transcriptional maintenance factor in morphogenesis
Abstract
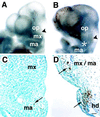
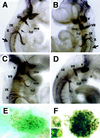
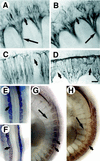
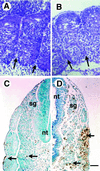
Determinative events in vertebrate embryogenesis appear to require the continuous expression of spatial regulators such as the clustered homeobox genes. The mechanisms that govern long-term patterns of gene expression are not well understood. In Drosophila, active and silent states of developmentally regulated loci are maintained by trithorax and Polycomb group. We have examined the developmental role of a mammalian homolog of trx and putative oncogene, Mll. Knockout mice reveal that Mll is required for maintenance of gene expression early in embryogenesis. Downstream targets of Mll including Hoxa7 are activated appropriately in the absence of Mll but require Mll for sustaining their expression. The Mll-/- phenotype manifests later in development and is characterized by branchial arch dysplasia and aberrant segmental boundaries of spinal ganglia and somites. Thus, Mll represents an essential mechanism of transcriptional maintenance in mammalian development, which functions in multiple morphogenetic processes.
Figures



References
-
- Noden D M. J Neurobiol. 1993;24:248–261. - PubMed
-
- Tam P P L, Trainor P A. Anat Embryol. 1994;189:275–305. - PubMed
-
- Kieny M, Mauger A, Sengel P. Dev Biol. 1972;28:142–161. - PubMed
-
- Beddington R S P, Püschel A W, Rashbass P. In: Postimplantation Development in the Mouse. Chadwick D J, Marsh J, editors. New York: Wiley; 1992. pp. 61–77.
-
- Deschamps J, Wijgerde M. Dev Biol. 1993;156:473–480. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

