The role of preassembled cytoplasmic complexes in assembly of flagellar dynein subunits
- PMID: 9725897
- PMCID: PMC25499
- DOI: 10.1091/mbc.9.9.2337
The role of preassembled cytoplasmic complexes in assembly of flagellar dynein subunits
Abstract
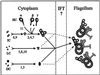
Previous work has revealed a cytoplasmic pool of flagellar precursor proteins capable of contributing to the assembly of new flagella, but how and where these components assemble is unknown. We tested Chlamydomonas outer-dynein arm subunit stability and assembly in the cytoplasm of wild-type cells and 11 outer dynein arm assembly mutant strains (oda1-oda11) by Western blotting of cytoplasmic extracts, or immunoprecipitates from these extracts, with five outer-row dynein subunit-specific antibodies. Western blots reveal that at least three oda mutants (oda6, oda7, and oda9) alter the level of a subunit that is not the mutant gene product. Immunoprecipitation shows that large preassembled flagellar complexes containing all five tested subunits (three heavy chains and two intermediate chains) exist within wild-type cytoplasm. When the preassembly of these subunits was examined in oda strains, we observed three patterns: complete coassembly (oda 1, 3, 5, 8, and 10), partial coassembly (oda7 and oda11), and no coassembly (oda2, 6, and 9) of the four tested subunits with HCbeta. Our data, together with previous studies, suggest that flagellar outer-dynein arms preassemble into a complete Mr approximately 2 x 10(6) dynein arm that resides in a cytoplasmic precursor pool before transport into the flagellar compartment.
Figures






References
-
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–254. - PubMed
-
- Burnette WN. Western blotting: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981;112:195–203. - PubMed
-
- Child FM, Apter MN. Experimental inhibition of ciliogenesis and ciliary regeneration in Arbacia embryo. Biol Bull. 1969;137:394–395.
-
- Cleveland DW, Fischer SG, Kirschner MW, Laemmli UK. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977;252:1102–1106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

