Differential expression and functions of cortical myosin IIA and IIB isotypes during meiotic maturation, fertilization, and mitosis in mouse oocytes and embryos
- PMID: 9725909
- PMCID: PMC25518
- DOI: 10.1091/mbc.9.9.2509
Differential expression and functions of cortical myosin IIA and IIB isotypes during meiotic maturation, fertilization, and mitosis in mouse oocytes and embryos
Abstract
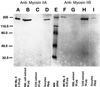
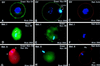
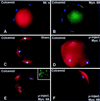
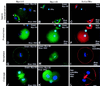
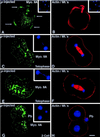
To explore the role of nonmuscle myosin II isoforms during mouse gametogenesis, fertilization, and early development, localization and microinjection studies were performed using monospecific antibodies to myosin IIA and IIB isotypes. Each myosin II antibody recognizes a 205-kDa protein in oocytes, but not mature sperm. Myosin IIA and IIB demonstrate differential expression during meiotic maturation and following fertilization: only the IIA isoform detects metaphase spindles or accumulates in the mitotic cleavage furrow. In the unfertilized oocyte, both myosin isoforms are polarized in the cortex directly overlying the metaphase-arrested second meiotic spindle. Cortical polarization is altered after spindle disassembly with Colcemid: the scattered meiotic chromosomes initiate myosin IIA and microfilament assemble in the vicinity of each chromosome mass. During sperm incorporation, both myosin II isotypes concentrate in the second polar body cleavage furrow and the sperm incorporation cone. In functional experiments, the microinjection of myosin IIA antibody disrupts meiotic maturation to metaphase II arrest, probably through depletion of spindle-associated myosin IIA protein and antibody binding to chromosome surfaces. Conversely, the microinjection of myosin IIB antibody blocks microfilament-directed chromosome scattering in Colcemid-treated mature oocytes, suggesting a role in mediating chromosome-cortical actomyosin interactions. Neither myosin II antibody, alone or coinjected, blocks second polar body formation, in vitro fertilization, or cytokinesis. Finally, microinjection of a nonphosphorylatable 20-kDa regulatory myosin light chain specifically blocks sperm incorporation cone disassembly and impedes cell cycle progression, suggesting that interference with myosin II phosphorylation influences fertilization. Thus, conventional myosins break cortical symmetry in oocytes by participating in eccentric meiotic spindle positioning, sperm incorporation cone dynamics, and cytokinesis. Although murine sperm do not express myosin II, different myosin II isotypes may have distinct roles during early embryonic development.
Figures






References
-
- Alexandre H, Van Cauwenberge A, Mulnard J. Involvement of microtubules and microfilaments in the control of the nuclear movement during maturation of mouse oocyte. Dev Biol. 1989;136:311–320. - PubMed
-
- Amsterdam A, Lindner AR, Groschel-Stewart U. Localization of actin and myosin in the rat oocyte and follicular wall by immunofluorescence. Anat Rec. 1976;187:311–328. - PubMed
-
- Battaglia DE, Gaddum-Rosse P. The distribution of polymerized actin in the rat egg and its sensitivity to cytochalasin B during fertilization. J Exp Zool. 1986;237:97–105. - PubMed
-
- Bavister BD. A consistently successful procedure for in vitro fertilization of golden hamster eggs. Gamete Res. 1989;23:139–158. - PubMed
-
- Campanella C, Gabbiani G, Baccetti B, Burrini AG, Pallini V. Actin and myosin in the vertebrate acrosomal region. J Submicrosc Cytol. 1979;11:53–71.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

