Rho and Rab small G proteins coordinately reorganize stress fibers and focal adhesions in MDCK cells
- PMID: 9725912
- PMCID: PMC25527
- DOI: 10.1091/mbc.9.9.2561
Rho and Rab small G proteins coordinately reorganize stress fibers and focal adhesions in MDCK cells
Abstract
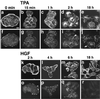
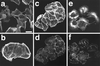
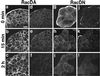
The Rho subfamily of the Rho small G protein family (Rho) regulates formation of stress fibers and focal adhesions in many types of cultured cells. In moving cells, dynamic and coordinate disassembly and reassembly of stress fibers and focal adhesions are observed, but the precise mechanisms in the regulation of these processes are poorly understood. We previously showed that 12-O-tetradecanoylphorbol-13-acetate (TPA) first induced disassembly of stress fibers and focal adhesions followed by their reassembly in MDCK cells. The reassembled stress fibers showed radial-like morphology that was apparently different from the original. We analyzed here the mechanisms of these TPA-induced processes. Rho inactivation and activation were necessary for the TPA-induced disassembly and reassembly, respectively, of stress fibers and focal adhesions. Both inactivation and activation of the Rac subfamily of the Rho family (Rac) inhibited the TPA-induced reassembly of stress fibers and focal adhesions but not their TPA-induced disassembly. Moreover, microinjection or transient expression of Rab GDI, a regulator of all the Rab small G protein family members, inhibited the TPA-induced reassembly of stress fibers and focal adhesions but not their TPA-induced disassembly, indicating that, furthermore, activation of some Rab family members is necessary for their TPA-induced reassembly. Of the Rab family members, at least Rab5 activation was necessary for the TPA-induced reassembly of stress fibers and focal adhesions. The TPA-induced, small G protein-mediated reorganization of stress fibers and focal adhesions was closely related to the TPA-induced cell motility. These results indicate that the Rho and Rab family members coordinately regulate the TPA-induced reorganization of stress fibers and focal adhesions that may cause cell motility.
Figures







Similar articles
-
Distinct actions and cooperative roles of ROCK and mDia in Rho small G protein-induced reorganization of the actin cytoskeleton in Madin-Darby canine kidney cells.Mol Biol Cell. 1999 Aug;10(8):2481-91. doi: 10.1091/mbc.10.8.2481. Mol Biol Cell. 1999. PMID: 10436006 Free PMC article.
-
Involvement of Cdc42 small G protein in cell-cell adhesion, migration and morphology of MDCK cells.Oncogene. 1999 Jul 8;18(27):3996-4006. doi: 10.1038/sj.onc.1202773. Oncogene. 1999. PMID: 10435623
-
Translocation of activated Rho from the cytoplasm to membrane ruffling area, cell-cell adhesion sites and cleavage furrows.Oncogene. 1995 Jul 6;11(1):39-48. Oncogene. 1995. PMID: 7624130
-
[Mechanisms of cell adhesion and migration].Gan To Kagaku Ryoho. 1999 Aug;26(9):1359-66. Gan To Kagaku Ryoho. 1999. PMID: 10478193 Review. Japanese.
-
The Rho small G protein family-Rho GDI system as a temporal and spatial determinant for cytoskeletal control.Biochem Biophys Res Commun. 1998 Apr 28;245(3):641-5. doi: 10.1006/bbrc.1998.8253. Biochem Biophys Res Commun. 1998. PMID: 9588168 Review.
Cited by
-
Vesicular Trafficking Defects, Developmental Abnormalities, and Alterations in the Cellular Death Process Occur in Cell Lines that Over-Express Dictyostelium GTPase, Rab2, and Rab2 Mutants.Biology (Basel). 2014 Aug 25;3(3):514-35. doi: 10.3390/biology3030514. Biology (Basel). 2014. PMID: 25157910 Free PMC article.
-
Angiostatin effects on endothelial cells mediated by ceramide and RhoA.EMBO Rep. 2001 Jun;2(6):536-40. doi: 10.1093/embo-reports/kve115. EMBO Rep. 2001. PMID: 11415988 Free PMC article.
-
Lamellipodium extension and membrane ruffling require different SNARE-mediated trafficking pathways.BMC Cell Biol. 2010 Aug 10;11:62. doi: 10.1186/1471-2121-11-62. BMC Cell Biol. 2010. PMID: 20698987 Free PMC article.
-
Real-time monitoring of hematopoietic cell interaction with fibronectin fragment: the effect of histone deacetylase inhibitors.Cell Adh Migr. 2013 May-Jun;7(3):275-82. doi: 10.4161/cam.24531. Epub 2013 Apr 8. Cell Adh Migr. 2013. PMID: 23567296 Free PMC article.
-
Phosphate-binding loop and Rab GTPase function: mutations at Ser29 and Ala30 of Rab5 lead to loss-of-function as well as gain-of-function phenotype.Biochem J. 2001 May 1;355(Pt 3):681-9. doi: 10.1042/bj3550681. Biochem J. 2001. PMID: 11311130 Free PMC article.
References
-
- Aktories K, Rösener S, Blaschke U, Chhatwal GS. Botulinum ADP-ribosyltransferase C3: purification of the enzyme and characterization of the ADP-ribosylation reaction in platelet membranes. Eur J Biochem. 1988;172:445–450. - PubMed
-
- Braun U, Habermann B, Just I, Aktories K, Vandekerckhove J. Purification of the 22kDa protein substrate of botulinum ADP-ribosyltransferase C3 from porcine brain cytosol and its characterization as a GTP-binding protein highly homologous to the rho gene product. FEBS Lett. 1989;243:70–76. - PubMed
-
- Bretcher MS. Getting membrane flow and the cytoskeleton to cooperate in moving cells. Cell. 1996;87:601–606. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

