Interactions between growth factors and integrins: latent forms of transforming growth factor-beta are ligands for the integrin alphavbeta1
- PMID: 9725916
- PMCID: PMC25536
- DOI: 10.1091/mbc.9.9.2627
Interactions between growth factors and integrins: latent forms of transforming growth factor-beta are ligands for the integrin alphavbeta1
Abstract
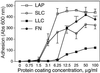
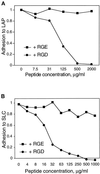
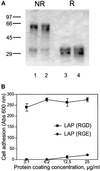
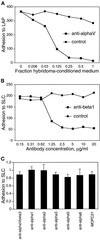
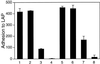
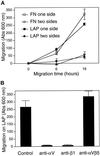
The multipotential cytokine transforming growth factor-beta (TGF-beta) is secreted in a latent form. Latency results from the noncovalent association of TGF-beta with its processed propeptide dimer, called the latency-associated peptide (LAP); the complex of the two proteins is termed the small latent complex. Disulfide bonding between LAP and latent TGF-beta-binding protein (LTBP) produces the most common form of latent TGF-beta, the large latent complex. The extracellular matrix (ECM) modulates the activity of TGF-beta. LTBP and the LAP propeptides of TGF-beta (isoforms 1 and 3), like many ECM proteins, contain the common integrin-binding sequence RGD. To increase our understanding of latent TGF-beta function in the ECM, we determined whether latent TGF-beta1 interacts with integrins. A549 cells adhered and spread on plastic coated with LAP, small latent complex, and large latent complex but not on LTBP-coated plastic. Adhesion was blocked by an RGD peptide, and cells were unable to attach to a mutant form of recombinant LAP lacking the RGD sequence. Adhesion was also blocked by mAbs to integrin subunits alphav and beta1. We purified LAP-binding integrins from extracts of A549 cells using LAP bound to Sepharose. alphavbeta1 eluted with EDTA. After purification in the presence of Mn2+, a small amount of alphavbeta5 was also detected. A549 cells migrated equally on fibronectin- and LAP-coated surfaces; migration on LAP was alphavbeta1 dependent. These results establish alphavbeta1 as a LAP-beta1 receptor. Interactions between latent TGF-beta and alphavbeta1 may localize latent TGF-beta to the surface of specific cells and may allow the TGF-beta1 gene product to initiate signals by both TGF-beta receptor and integrin pathways.
Figures


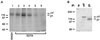
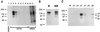
References
-
- Bodary SC, McLean JW. The integrin β1 subunit associates with the vitronectin receptor αv subunit to form a novel vitronectin receptor in a human embryonic kidney cell line. J Biol Chem. 1990;265:5938–5941. - PubMed
-
- Brooks PC, Stromblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP, Cheresh DA. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin αvβ3. Cell. 1996;85:683–693. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

