Contribution of indole-3-acetic acid production to the epiphytic fitness of erwinia herbicola
- PMID: 9726868
- PMCID: PMC106718
- DOI: 10.1128/AEM.64.9.3256-3263.1998
Contribution of indole-3-acetic acid production to the epiphytic fitness of erwinia herbicola
Abstract
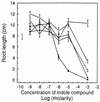
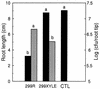
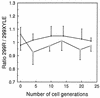
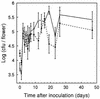
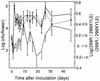
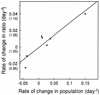
Erwinia herbicola 299R produces large quantities of indole-3-acetic acid (IAA) in culture media supplemented with L-tryptophan. To assess the contribution of IAA production to epiphytic fitness, the population dynamics of the wild-type strain and an IAA-deficient mutant of this strain on leaves were studied. Strain 299XYLE, an isogenic IAA-deficient mutant of strain 299R, was constructed by insertional interruption of the indolepyruvate decarboxylase gene of strain 299R with the xylE gene, which encodes a 2,3-catechol dioxygenase from Pseudomonas putida mt-2. The xylE gene provided a useful marker for monitoring populations of the IAA-deficient mutant strain in mixed populations with the parental strain in ecological studies. A root bioassay for IAA, in which strain 299XYLE inhibited significantly less root elongation than strain 299R, provided evidence that E. herbicola produces IAA on plant surfaces in amounts sufficient to affect the physiology of its host and that IAA production in strain 299R is not solely an in vitro phenomenon. The epiphytic fitness of strains 299R and 299XYLE was evaluated in greenhouse and field studies by analysis of changes in the ratio of the population sizes of these two strains after inoculation as mixtures onto plants. Populations of the parental strain increased to approximately twice those of the IAA-deficient mutant strain after coinoculation in a proportion of 1:1 onto bean plants in the greenhouse and onto pear flowers in field studies. In all experiments, the ratio of the population sizes of strain 299R and 299XYLE increased during periods of active growth on plant tissue but not when population sizes were not increasing with time.
Figures



References
-
- Brandl M, Clark E M, Lindow S E. Characterization of the indole-3-acetic acid (IAA) biosynthetic pathway in an epiphytic strain of Erwinia herbicola and IAA production in vitro. Can J Microbiol. 1996;42:586–592.
-
- Brandl M T, Lindow S E. Environmental signals modulate the expression of an indole-3-acetic acid biosynthetic gene in Erwinia herbicola. Mol Plant-Microbe Interact. 1997;10:499–505.
-
- Chet I, Zilberstein Y, Henis Y. Chemotaxis of Pseudomonas lachrymans to plant extracts and to water droplets collected from the leaf surface of resistant and susceptible plants. Physiol Plant Pathol. 1973;3:473–479.
-
- Costacurta A, Vanderleyden J. Synthesis of phytohormones by plant-associated bacteria. Crit Rev Microbiol. 1995;21:1–18. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

