Human immunodeficiency virus type 1-like DNA sequences and immunoreactive viral particles with unique association with breast cancer
- PMID: 9729531
- PMCID: PMC95635
- DOI: 10.1128/CDLI.5.5.645-653.1998
Human immunodeficiency virus type 1-like DNA sequences and immunoreactive viral particles with unique association with breast cancer
Abstract
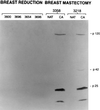
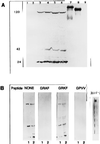
RAK antigens p120, p42, and p25 exhibit molecular and immunological similarity to the proteins encoded by human immunodeficiency virus type 1 (HIV-1) and are expressed by 95% of breast and gynecological cancer cases in women and prostate cancer cases in men. The binding of an epitope-specific anti-HIV-1 gp120 monoclonal antibody (MAb) (amino acids 308 to 322) to cancer RAK antigens has been found to be inhibited by a peptide derived from variable loop V3 of HIV-1. Breast cancer DNAs of 40 patients were PCR amplified with HIV-1 gp41-derived primers, and all of the samples were found to be positive. The DNA fragments amplified in seven blindly selected breast cancer samples were sequenced. The breast cancer DNA sequences showed at least 90% homology to the HIV-1 gene for gp41. Antisense oligonucleotides complementary to the HIV-1-like sequences inhibited reverse transcriptase activity and inhibited the growth of breast cancer cells in vitro. Viral particles detected in breast cancer cell lines were strongly immunogold labeled with the anti-HIV-1 gp120 MAb. The results obtained strongly suggest that the long-postulated breast cancer virus may, in fact, be related to HIV-1.
Figures








References
-
- Andersson M L, Medstrand P, Yin H, Blomberg J. Differential expression of human endogenous retroviral sequences similar to mouse mammary tumor virus in normal peripheral blood mononuclear cells. AIDS Res Hum Retroviruses. 1996;12:833–840. - PubMed
-
- Biesecker B B, Boehnke M, Calzone K, Markel D S, Garber J E, Collins F S, Weber B L. Genetic counseling for families with inherited susceptibility to breast and ovarian cancer. JAMA. 1993;269:1970–1974. - PubMed
-
- Bouton A H, Parsons J T. Retroviruses and cancer: models for cancer in animals and humans. Cancer Invest. 1993;11:70–79. - PubMed
-
- Chang Y, Cesarman E, Pessin M S, Lee F, Culpepper J, Knowles D M, Moore P S. Identification of herpes virus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865–1869. - PubMed
-
- Chopra H C, Feller W F. Virus-like particles in human breast cancer. Tex Rep Biol Med. 1969;27:945–954. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

