Role of arachidonic acid and its metabolites in the priming of NADPH oxidase in human polymorphonuclear leukocytes by peritoneal dialysis effluent
- PMID: 9729536
- PMCID: PMC95640
- DOI: 10.1128/CDLI.5.5.683-689.1998
Role of arachidonic acid and its metabolites in the priming of NADPH oxidase in human polymorphonuclear leukocytes by peritoneal dialysis effluent
Abstract
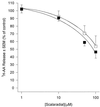
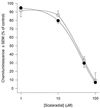
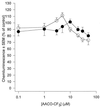
Peritoneal dialysis effluent (PDE) contains a low-molecular-weight solute that will activate and prime the NADPH oxidase of human neutrophils via a phospholipase A2 (PLA2)-dependent mechanism. Since the products of PLA2 are known to activate and prime the oxidase we have investigated their role in the dialysis effluent-mediated activation and priming of human neutrophils. NADPH oxidase activity of PDE-primed and -unprimed neutrophils was measured by lucigenin-enhanced chemiluminescence in the presence of known inhibitors of the arachidonic acid cascade. Incubation of neutrophils with the nonselective PLA2 inhibitor quinacrine (0 to 100 microM) reduced oxidase activity in both primed and unprimed cells. Furthermore, primed cells were more sensitive to the action of quinacrine than were unprimed cells. We were unable to determine the relative roles of secretory PLA2 (sPLA2) and cytosolic PLA2 (cPLA2) since the selective sPLA2 inhibitor scalaradial (0 to 100 microM) inhibited oxidase activity in both groups of cells by similar degrees, while the specific cPLA2 inhibitor AACO-CF3 (0 to 50 microM) failed to affect activity in either group. Inhibition of platelet-activating factor (PAF), cycloxygenase, and 5-lipoxygenase-activating protein by hexanolamino-PAF (0 to 25 microM), flurbiprofen (0 to 25 microM), and MK886 (0 to 5 microM), respectively, had no effect upon oxidase activity. However, the direct inhibition of 5-lipoxygenase by caffeic acid or lipoxin A4 resulted in a similar concentration-dependent attenuation of oxidase activity in both primed and unprimed cells. Leukotriene B4 (LTB4) release from primed neutrophils was comparable to that from unprimed cells with the exception of phorbol myristate acetate-stimulated cells, which released fivefold more LTB4 than control. Taken together, these results suggest that it is arachidonic acid per se, and not its metabolites, that is important in priming of the neutrophil NADPH oxidase by dialysis effluent.
Figures




References
-
- Bates E J, Harvey D P, Ferrante A. Inhibition of neutrophil respiratory burst and degranulation responses to platelet-activating factor by antagonists WEB 2086, CV 6209 and CV 3988. Int Arch Allergy Immunol. 1992;97:50–56. - PubMed
-
- Bauldry S A, McCall C E, Cousart S L, Bass D A. TNFα priming of PLA2 activation in human neutrophils—an alternative mechanism of priming. J Immunol. 1991;149:1277–1285. - PubMed
-
- Bauldry S A, Wykle R L, Bass D A. PLA2 activation in human neutrophils. J Biol Chem. 1988;263:16787–16795. - PubMed
-
- Boyum, A. 1968. Isolation of mononuclear cells and granulocytes from human blood. Scand. J. Clin. Lab. Investig. 21(Suppl. 97):77–89. - PubMed
-
- Classon H-E, Lundberg U, Malmsten C. Serum coated zymosan stimulates the synthesis of LTB4 in human PMN. Inhibition by cAMP. Biochem Biophys Res Commun. 1981;99:1230–1237. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

