Neutrophil chemiluminescence during phagocytosis is inhibited by abnormally elevated levels of acetoacetate: implications for diabetic susceptibility to infections
- PMID: 9729546
- PMCID: PMC95650
- DOI: 10.1128/CDLI.5.5.740-743.1998
Neutrophil chemiluminescence during phagocytosis is inhibited by abnormally elevated levels of acetoacetate: implications for diabetic susceptibility to infections
Abstract
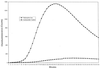
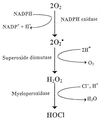
Human neutrophils by a chemiluminescence assay exhibit diminished phagocytic activity in the presence of abnormally high levels of the serum metabolite acetoacetate. These findings, along with our previous evidence demonstrating myeloperoxidase inhibition by acetoacetate, implicate metabolic ketosis in the inhibition of neutrophil microbicidal activity and thus in increased susceptibility to infections.
Figures


References
-
- Harrison J E, Saeed F A. Acetoacetate is an electron donor to myeloperoxidase and a promoter of myeloperoxidase-catalyzed fatty acid peroxidation. Biochem Med. 1981;26:339–355. - PubMed
-
- Harrison J E, Saeed F A. ACS Annual Meeting Abstracts. 1981. Acetoacetate: an electron donor and halogen acceptor for neutrophil myeloperoxidase and a promoter of fatty acid peroxidation. - PubMed
-
- Harrison J E, Saeed F A. Radical acetoacetate oxidation by myeloperoxidase, lactoperoxidase, prostaglandin synthetase, and prostacyclin synthetase: implications for atherosclerosis. Biochem Med. 1983;29:149–163. - PubMed
-
- Harrison J E, Schultz J. Studies on the chlorinating mechanism of myeloperoxidase. J Biol Chem. 1976;251:1371–1374. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

