Chlorophyll fluorescence analysis of cyanobacterial photosynthesis and acclimation
- PMID: 9729605
- PMCID: PMC98930
- DOI: 10.1128/MMBR.62.3.667-683.1998
Chlorophyll fluorescence analysis of cyanobacterial photosynthesis and acclimation
Abstract
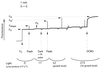
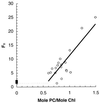
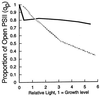
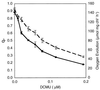
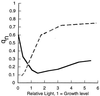
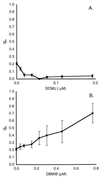
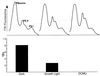
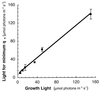
Cyanobacteria are ecologically important photosynthetic prokaryotes that also serve as popular model organisms for studies of photosynthesis and gene regulation. Both molecular and ecological studies of cyanobacteria benefit from real-time information on photosynthesis and acclimation. Monitoring in vivo chlorophyll fluorescence can provide noninvasive measures of photosynthetic physiology in a wide range of cyanobacteria and cyanolichens and requires only small samples. Cyanobacterial fluorescence patterns are distinct from those of plants, because of key structural and functional properties of cyanobacteria. These include significant fluorescence emission from the light-harvesting phycobiliproteins; large and rapid changes in fluorescence yield (state transitions) which depend on metabolic and environmental conditions; and flexible, overlapping respiratory and photosynthetic electron transport chains. The fluorescence parameters FV/FM, FV'/FM',qp,qN, NPQ, and phiPS II were originally developed to extract information from the fluorescence signals of higher plants. In this review, we consider how the special properties of cyanobacteria can be accommodated and used to extract biologically useful information from cyanobacterial in vivo chlorophyll fluorescence signals. We describe how the pattern of fluorescence yield versus light intensity can be used to predict the acclimated light level for a cyanobacterial population, giving information valuable for both laboratory and field studies of acclimation processes. The size of the change in fluorescence yield during dark-to-light transitions can provide information on respiration and the iron status of the cyanobacteria. Finally, fluorescence parameters can be used to estimate the electron transport rate at the acclimated growth light intensity.
Figures


References
-
- Adams W W, Demmig-Adams B. Energy dissipation and photoprotection in leaves of higher plants. In: Yamamoto H Y, Smith C M, editors. Photosynthetic responses to the environment. Rockville, Md: American Society of Plant Physiologists; 1993. pp. 27–36.
-
- Allen J F, Sanders C E, Holmes N G. Correlation of membrane protein phosphorylation with excitation energy distribution in the cyanobacterium Synechococcus 6301. FEBS Lett. 1985;193:271–275.
-
- Asada K, Kiso K, Yoshikawa K. Univalent reduction of molecular oxygen by spinach chloroplasts on illumination. J Biol Chem. 1974;249:2175–2181. - PubMed
-
- Badger M R, Price G D. The CO2 concentrating mechanism in cyanobacteria and green algae. Physiol Plant. 1992;84:606–615.
-
- Badger M R, Schreiber U. Effects of inorganic carbon accumulation on photosynthetic oxygen reduction and cyclic electron flow in the cyanobacterium Synechococcus PCC 7942. Photosynth Res. 1993;37:177–191. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

