The specificity of peptides bound to human histocompatibility leukocyte antigen (HLA)-B27 influences the prevalence of arthritis in HLA-B27 transgenic rats
- PMID: 9730889
- PMCID: PMC2213380
- DOI: 10.1084/jem.188.5.877
The specificity of peptides bound to human histocompatibility leukocyte antigen (HLA)-B27 influences the prevalence of arthritis in HLA-B27 transgenic rats
Erratum in
- J Exp Med 2000 Feb 21;191(4):following 746
Abstract
Human histocompatibility leukocyte antigen B27 is highly associated with the rheumatic diseases termed spondyloarthropathies, but the mechanism is not known. B27 transgenic rats develop a spontaneous disease resembling the human spondyloarthropathies that includes arthritis and colitis. To investigate whether this disease requires the binding of specific peptides to B27, we made a minigene construct in which a peptide from influenza nucleoprotein, NP383-391 (SRYWAIRTR), which binds B27 with high affinity, is targeted directly to the ER by the signal peptide of the adenovirus E3/gp19 protein. Rats transgenic for this minigene, NP1, were made and bred with B27 rats. The production of the NP383-391 peptide in B27(+)NP1(+) rats was confirmed immunologically and by mass spectrometry. The NP1 product displaced approximately 90% of the 3H-Arg-labeled endogenous peptide fraction in B27(+)NP1(+) spleen cells. Male B27(+)NP1(+) rats had a significantly reduced prevalence of arthritis, compared with B27(+)NP- males or B27(+) males with a control construct, NP2, whereas colitis was not significantly affected by the NP1 transgene. These findings support the hypothesis that B27-related arthritis requires binding of a specific peptide or set of peptides to B27, and they demonstrate a method for efficient transgenic targeting of peptides to the ER.
Figures

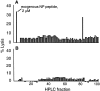


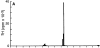



Comment in
-
Findings.NIH Guide Grants Contracts (Bethesda). 2000 Sep 12:OD-00-052. NIH Guide Grants Contracts (Bethesda). 2000. PMID: 12458560 Free PMC article. No abstract available.
References
-
- López de Castro JA. The pathogenetic role of HLA-B27 in chronic arthritis. Curr Opin Immunol. 1998;10:59–66. - PubMed
-
- Taurog, J.D. 1998. HLA-B27 subtypes, disease susceptibility, and peptide binding specificity. In The Spondylarthritides. A. Calin and J.D. Taurog, editors. Oxford University Press, Oxford, UK. 267–274.
-
- Townsend A, Bodmer H. Antigen recognition by class I-restricted T lymphocytes. Annu Rev Immunol. 1989;7:601–632. - PubMed
-
- Lehner PJ, Cresswell P. Processing and delivery of peptides presented by MHC class I molecules. Curr Opin Immunol. 1996;8:59–67. - PubMed
-
- Benjamin R, Parham P. Guilt by association: HLA-B27 and ankylosing spondylitis. Immunol Today. 1990;11:137–142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

