TRANCE is necessary and sufficient for osteoblast-mediated activation of bone resorption in osteoclasts
- PMID: 9730902
- PMCID: PMC2213394
- DOI: 10.1084/jem.188.5.997
TRANCE is necessary and sufficient for osteoblast-mediated activation of bone resorption in osteoclasts
Abstract
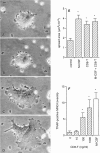
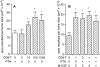
TRANCE (tumor necrosis factor-related activation-induced cytokine) is a recently described member of the tumor necrosis factor superfamily that stimulates dendritic cell survival and has also been found to induce osteoclastic differentiation from hemopoietic precursors. However, its effects on mature osteoclasts have not been defined. It has long been recognized that stimulation of osteoclasts by agents such as parathyroid hormone (PTH) occurs through a hormonal interaction with osteoblastic cells, which are thereby induced to activate osteoclasts. To determine whether TRANCE accounts for this activity, we tested its effects on mature osteoclasts. TRANCE rapidly induced a dramatic change in osteoclast motility and spreading and inhibited apoptosis. In populations of osteoclasts that were unresponsive to PTH, TRANCE caused activation of bone resorption equivalent to that induced by PTH in the presence of osteoblastic cells. Moreover, osteoblast-mediated stimulation of bone resorption was abrogated by soluble TRANCE receptor and by the soluble decoy receptor osteoprotegerin (OPG), and stimulation of isolated osteoclasts by TRANCE was neutralized by OPG. Thus, TRANCE expression by osteoblasts appears to be both necessary and sufficient for hormone-mediated activation of mature osteoclasts, and TRANCE-R is likely to be a receptor for signal transduction for activation of the osteoclast and its survival.
Figures
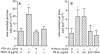
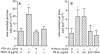
References
-
- Chambers, T.J. 1992. Regulation of osteoclast development and function. In Biology and Physiology of the Osteoclast. B.R. Rifkin and C.V. Gay, editors. CRC Press, Boca Raton, FL. 105–128.
-
- Suda T, Takahashi N, Martin TJ. Modulation of osteoclast differentiation. Endocr Rev. 1992;13:66–79. - PubMed
-
- Wong BR, Rho J, Arron J, Robinson E, Orlinick J, Chao M, Kalachikov S, Cayani E, Bartlett FS, III, Frankel WN, et al. TRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-jun N-terminal kinase in T cells. J Biol Chem. 1997;272:25190–25194. - PubMed
-
- Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, DuBose RF, Cosman D, Galibert L. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390:175–179. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

