The yeast V159N actin mutant reveals roles for actin dynamics in vivo
- PMID: 9732289
- PMCID: PMC2149338
- DOI: 10.1083/jcb.142.5.1289
The yeast V159N actin mutant reveals roles for actin dynamics in vivo
Abstract
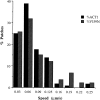
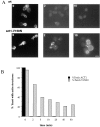
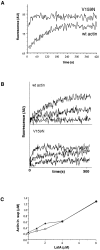
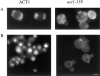
Actin with a Val 159 to Asn mutation (V159N) forms actin filaments that depolymerize slowly because of a failure to undergo a conformational change after inorganic phosphate release. Here we demonstrate that expression of this actin results in reduced actin dynamics in vivo, and we make use of this property to study the roles of rapid actin filament turnover. Yeast strains expressing the V159N mutant (act1-159) as their only source of actin have larger cortical actin patches and more actin cables than wild-type yeast. Rapid actin dynamics are not essential for cortical actin patch motility or establishment of cell polarity. However, fluid phase endocytosis is defective in act1-159 strains. act1-159 is synthetically lethal with cofilin and profilin mutants, supporting the conclusion that mutations in all of these genes impair the polymerization/ depolymerization cycle. In contrast, act1-159 partially suppresses the temperature sensitivity of a tropomyosin mutant, and the loss of cytoplasmic cables seen in fimbrin, Mdm20p, and tropomyosin null mutants, suggesting filament stabilizing functions for these actin-binding proteins. Analysis of the cables in these double-mutant cells supports a role for fimbrin in organizing cytoplasmic cables and for Mdm20p and tropomyosin in excluding cofilin from the cables.
Figures




References
-
- Adams AE, Botstein D, Drubin DG. Requirement of yeast fimbrin for actin organization and morphogenesis in vivo. Nature. 1991;354:404–408. - PubMed
-
- Berstein BW, Bamburg JR. Tropomyosin binding to F-actin protects the F-actin from disassembly by brain actin-depolymerizing-factor (ADF) Cell Motil. 1982;2:1–8. - PubMed
-
- Carlier MF. Actin polymerization and ATP hydrolysis. Adv Biophys. 1990;26:51–73. - PubMed
-
- Carlier MF, Pantaloni D. Direct evidence for ADP-Pi-F-actin as the major intermediate in ATP-actin polymerization. Rate of dissociation of Pi from actin filaments. Biochemistry. 1986;25:7789–7792. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

