Involvement of an actomyosin contractile ring in Saccharomyces cerevisiae cytokinesis
- PMID: 9732290
- PMCID: PMC2149343
- DOI: 10.1083/jcb.142.5.1301
Involvement of an actomyosin contractile ring in Saccharomyces cerevisiae cytokinesis
Abstract
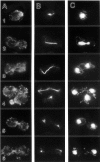
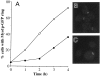
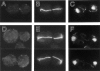
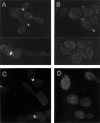
In Saccharomyces cerevisiae, the mother cell and bud are connected by a narrow neck. The mechanism by which this neck is closed during cytokinesis has been unclear. Here we report on the role of a contractile actomyosin ring in this process. Myo1p (the only type II myosin in S. cerevisiae) forms a ring at the presumptive bud site shortly before bud emergence. Myo1p ring formation depends on the septins but not on F-actin, and preexisting Myo1p rings are stable when F-actin is depolymerized. The Myo1p ring remains in the mother-bud neck until the end of anaphase, when a ring of F-actin forms in association with it. The actomyosin ring then contracts to a point and disappears. In the absence of F-actin, the Myo1p ring does not contract. After ring contraction, cortical actin patches congregate at the mother-bud neck, and septum formation and cell separation rapidly ensue. Strains deleted for MYO1 are viable; they fail to form the actin ring but show apparently normal congregation of actin patches at the neck. Some myo1Delta strains divide nearly as efficiently as wild type; other myo1Delta strains divide less efficiently, but it is unclear whether the primary defect is in cytokinesis, septum formation, or cell separation. Even cells lacking F-actin can divide, although in this case division is considerably delayed. Thus, the contractile actomyosin ring is not essential for cytokinesis in S. cerevisiae. In its absence, cytokinesis can still be completed by a process (possibly localized cell-wall synthesis leading to septum formation) that appears to require septin function and to be facilitated by F-actin.
Figures





References
-
- Adams, A.E.M. 1984. Cellular morphogenesis in the yeast Saccharomyces cerevisiae. Ph.D. thesis. University of Michigan, Ann Arbor, MI. 179 pp.
-
- Alfa CE, Hyams JS. Distribution of tubulin and actin through the cell division cycle of the fission yeast Schizosaccharomyces japonicus var. versatilis: a comparison with Schizosaccharomyces pombe. . J Cell Sci. 1990;96:71–77. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

