Mode of action of interleukin-6 on mature osteoclasts. Novel interactions with extracellular Ca2+ sensing in the regulation of osteoclastic bone resorption
- PMID: 9732294
- PMCID: PMC2149353
- DOI: 10.1083/jcb.142.5.1347
Mode of action of interleukin-6 on mature osteoclasts. Novel interactions with extracellular Ca2+ sensing in the regulation of osteoclastic bone resorption
Abstract
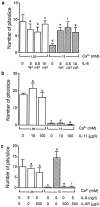
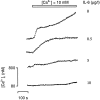
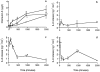
We describe a physiologically significant mechanism through which interleukin-6 (IL-6) and a rising ambient Ca2+ interact to regulate osteoclastic bone resorption. VOXEL-based confocal microscopy of nonpermeabilized osteoclasts incubated with anti- IL-6 receptor antibodies revealed intense, strictly peripheral plasma membrane fluorescence. IL-6 receptor expression in single osteoclasts was confirmed by in situ reverse transcriptase PCR histochemistry. IL-6 (5 ng/l to 10 microg/l), but not IL-11 (10 and 100 microg/l), reversed the inhibition of osteoclastic bone resorption induced by high extracellular Ca2+ (15 mM). The IL-6 effect was abrogated by excess soluble IL-6 receptor (500 microg/l). Additionally, IL-6 (5 pg/l to 10 microg/l) inhibited cytosolic Ca2+ signals triggered by high Ca2+ or Ni2+. In separate experiments, osteoclasts incubated in 10 mM Ca2+ or on bone released more IL-6 than those in 1.25 mM Ca2+. Furthermore, IL-6 mRNA histostaining was more intense in osteoclasts in 10 or 20 mM Ca2+ than cells in 1.25 mM Ca2+. Similarly, IL-6 receptor mRNA histostaining was increased in osteoclasts incubated in 5 or 10 mM Ca2+. Thus, while high Ca2+ enhances IL-6 secretion, the released IL-6 attenuates Ca2+ sensing and reverses inhibition of resorption by Ca2+. Such an autocrine-paracrine loop may sustain osteoclastic activity in the face of an inhibitory Ca2+ level generated locally during resorption.
Figures

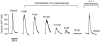

References
-
- Adebanjo OA, Pazianas M, Zaidi A, Shankar VS, Bascal ZA, Dacke CG, Huang CL-H, Zaidi M. Quantitative studies on the effect of prostacyclin on freshly isolated rat osteoclasts in culture. J Endocrinol. 1994;143:375–381. - PubMed
-
- Bellido T, Borba VZ, Robertson P, Manolagas SC. Activation of the Janus/STAT (signal transducer and activator of transcription) signal transduction pathway by interleukin-6-type cytokines promotes osteoblast differentiation. Endocrinology. 1997;138:3666–3676. - PubMed
-
- Boyde A, Ali NN, Jones SJ. Resorption of dentine by isolated osteoclasts in vitro. . Br Dental J. 1984;156:216–220. - PubMed
-
- Caputo C. Nickel substitution for calcium and the timecourse of potassium conductances of single muscle fibres. J Muscle Res Cell Motil. 1981;2:167–182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

