Alterations in the cytoskeleton accompany aluminum-induced growth inhibition and morphological changes in primary roots of maize
- PMID: 9733535
- PMCID: PMC34852
- DOI: 10.1104/pp.118.1.159
Alterations in the cytoskeleton accompany aluminum-induced growth inhibition and morphological changes in primary roots of maize
Abstract
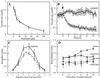
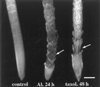
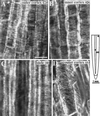
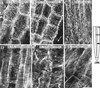
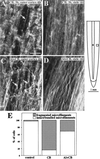
Although Al is one of the major factors limiting crop production, the mechanisms of toxicity remain unknown. The growth inhibition and swelling of roots associated with Al exposure suggest that the cytoskeleton may be a target of Al toxicity. Using indirect immunofluorescence microscopy, microtubules and microfilaments in maize (Zea mays L.) roots were visualized and changes in their organization and stability correlated with the symptoms of Al toxicity. Growth studies showed that the site of Al toxicity was associated with the elongation zone. Within this region, Al resulted in a reorganization of microtubules in the inner cortex. However, the orientation of microtubules in the outer cortex and epidermis remained unchanged even after chronic symptoms of toxicity were manifest. Auxin-induced reorientation and cold-induced depolymerization of microtubules in the outer cortex were blocked by Al pretreatment. These results suggest that Al increased the stability of microtubules in these cells. The stabilizing effect of Al in the outer cortex coincided with growth inhibition. Reoriented microfilaments were also observed in Al-treated roots, and Al pretreatment minimized cytochalasin B-induced microfilament fragmentation. These data show that reorganization and stabilization of the cytoskeleton are closely associated with Al toxicity in maize roots.
Figures





References
-
- Alfano F, Russel A, Gambardella R, Duckett JG. The actin cytoskeleton of the liverwort Riccia fluitans: effects of cytochalasin B and aluminum on rhizoid tip growth. J Plant Physiol. 1993;142:569–574.
-
- Andersland J, Fisher D, Wymer C, Cyr R, Parthasarathy M. Characterization of a monoclonal antibody prepared against plant actin. Cell Motil Cytoskel. 1994;29:339–344. - PubMed
-
- Baluska F, Barlow PW, Volkmann D. Complete disintegration of the microtubular cytoskeleton precedes its auxin-mediated reconstruction in postmitotic maize root cells. Plant Cell Physiol. 1996;37:1013–1021. - PubMed
-
- Baluska F, Brailsford RW, Hauskrecht M, Jackson MB, Barlow PW. Cellular dimorphism in the maize root cortex: involvement of microtubules, ethylene and gibberellin in the differentiation of cellular behavior in postmitotic growth zones. Bot Acta. 1993a;106:394–403.
-
- Baluska F, Parker JS, Barlow PW. The microtubular cytoskeleton in cells of cold-treated roots of maize (Zea mays L.) shows tissue-specific responses. Protoplasma. 1993b;172:84–96.
LinkOut - more resources
Full Text Sources

