Influence of Lif, the lysostaphin immunity factor, on acceptors of surface proteins and cell wall sorting efficiency in Staphylococcus carnosus
- PMID: 9733703
- PMCID: PMC107525
- DOI: 10.1128/JB.180.18.4960-4962.1998
Influence of Lif, the lysostaphin immunity factor, on acceptors of surface proteins and cell wall sorting efficiency in Staphylococcus carnosus
Abstract
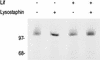
Proteins harboring a C-terminal cell wall sorting signal are covalently linked to pentaglycine acceptors within the staphylococcal peptidoglycan. This pentaglycine was modified when the lysostaphin immunity factor (Lif) of Staphylococcus simulans was expressed in Staphylococcus carnosus, likely by the exchange of two glycine residues for serine residues. A reporter protein was efficiently linked to the modified acceptor, indicating that the sorting reaction is not strictly dependent on the wild-type structures of the acceptors.
Figures

Similar articles
-
epr, which encodes glycylglycine endopeptidase resistance, is homologous to femAB and affects serine content of peptidoglycan cross bridges in Staphylococcus capitis and Staphylococcus aureus.J Bacteriol. 1997 Jul;179(13):4311-8. doi: 10.1128/jb.179.13.4311-4318.1997. J Bacteriol. 1997. PMID: 9209049 Free PMC article.
-
Studies on prolysostaphin processing and characterization of the lysostaphin immunity factor (Lif) of Staphylococcus simulans biovar staphylolyticus.Mol Microbiol. 1997 Mar;23(6):1251-65. doi: 10.1046/j.1365-2958.1997.2911657.x. Mol Microbiol. 1997. PMID: 9106216
-
The lysostaphin endopeptidase resistance gene (epr) specifies modification of peptidoglycan cross bridges in Staphylococcus simulans and Staphylococcus aureus.Appl Environ Microbiol. 1995 Apr;61(4):1475-9. doi: 10.1128/aem.61.4.1475-1479.1995. Appl Environ Microbiol. 1995. PMID: 7747966 Free PMC article.
-
Composition of bacterial cell walls in relation to antibiotic action.Adv Pharmacol (1962). 1969;7:283-307. Adv Pharmacol (1962). 1969. PMID: 4906304 Review. No abstract available.
-
Cell wall hydrolases and antibiotics: exploiting synergy to create efficacious new antimicrobial treatments.Curr Opin Microbiol. 2016 Oct;33:18-24. doi: 10.1016/j.mib.2016.05.006. Epub 2016 May 31. Curr Opin Microbiol. 2016. PMID: 27257994 Review.
Cited by
-
MpsAB is important for Staphylococcus aureus virulence and growth at atmospheric CO2 levels.Nat Commun. 2019 Aug 9;10(1):3627. doi: 10.1038/s41467-019-11547-5. Nat Commun. 2019. PMID: 31399577 Free PMC article.
-
Immunomimetic Designer Cells Protect Mice from MRSA Infection.Cell. 2018 Jul 12;174(2):259-270.e11. doi: 10.1016/j.cell.2018.05.039. Epub 2018 Jun 21. Cell. 2018. PMID: 29937224 Free PMC article.
-
Bioluminescence and 19F magnetic resonance imaging visualize the efficacy of lysostaphin alone and in combination with oxacillin against Staphylococcus aureus in murine thigh and catheter-associated infection models.Antimicrob Agents Chemother. 2014;58(3):1630-8. doi: 10.1128/AAC.01422-13. Epub 2013 Dec 23. Antimicrob Agents Chemother. 2014. PMID: 24366730 Free PMC article.
-
Anti-staphylococcal activities of lysostaphin and LytM catalytic domain.BMC Microbiol. 2012 Jun 6;12:97. doi: 10.1186/1471-2180-12-97. BMC Microbiol. 2012. PMID: 22672475 Free PMC article.
-
Staphylococcus aureus Stress Response to Bicarbonate Depletion.Int J Mol Sci. 2024 Aug 26;25(17):9251. doi: 10.3390/ijms25179251. Int J Mol Sci. 2024. PMID: 39273203 Free PMC article.
References
-
- Browder H P, Zygmunt J R, Young J R, Tavormina P A. Lysostaphin: enzymatic mode of action. Biochem Biophys Res Commun. 1965;19:383–389. - PubMed
-
- Götz F. Staphylococcus carnosus: a new host organism for gene cloning and protein production. Soc Appl Bacteriol Symp Ser. 1990;19:495–535. - PubMed
-
- Götz F, Schumacher B. Improvements of protoplast transformation in Staphylococcus carnosus. FEMS Microbiol Lett. 1987;40:285–288.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

