Dendritic cells route human immunodeficiency virus to lymph nodes after vaginal or intravenous administration to mice
- PMID: 9733818
- PMCID: PMC110098
- DOI: 10.1128/JVI.72.10.7822-7829.1998
Dendritic cells route human immunodeficiency virus to lymph nodes after vaginal or intravenous administration to mice
Abstract
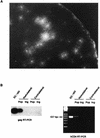
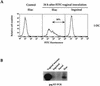
We have developed a murine model to study the involvement of dendritic cells (DC) in human immunodeficiency virus (HIV) routing from an inoculation site to the lymph nodes (LN). Murine bone marrow-derived DC migrate to the draining LN within 24 h after subcutaneous injection. After incubation of these cells with heat-inactivated (Hi) HIV type 1 (HIV-1), HIV RNA sequences were detected in the draining LN only. Upon injection of DC pulsed with infectious HIV, the virus recovered in the draining LN was still able to productively infect human T cells. After a vaginal challenge with Hi HIV-1, the virus could be detected in the iliac and sacral draining LN at 24 h after injection. After an intravenous challenge, the virus could be detected in peripheral LN as soon as 30 min after injection. The specific depletion of a myeloid-related LN DC population, previously shown to take up blood macromolecules and to translocate them into the LN, prevented HIV transport to LN. Together, our data demonstrate the critical role of DC for HIV routing to LN after either a vaginal or an intravenous challenge, which does not require their infection. Therefore, despite the fact that the mouse is not infectable by HIV, this small animal model might be useful to test preventive strategies against HIV.
Figures
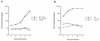


Similar articles
-
Analysis of mouse dendritic cell migration in vivo upon subcutaneous and intravenous injection.Immunology. 1999 Oct;98(2):181-8. doi: 10.1046/j.1365-2567.1999.00850.x. Immunology. 1999. PMID: 10540216 Free PMC article.
-
Murine Cytomegalovirus Spreads by Dendritic Cell Recirculation.mBio. 2017 Oct 3;8(5):e01264-17. doi: 10.1128/mBio.01264-17. mBio. 2017. PMID: 28974616 Free PMC article.
-
Comparison of CTL reactivity in the spleen and draining lymph nodes after immunization with peptides pulsed on dendritic cells or mixed with Freund's incomplete adjuvant.Immunol Lett. 2003 Nov 15;90(1):13-8. doi: 10.1016/s0165-2478(03)00158-5. Immunol Lett. 2003. PMID: 14611902
-
Bone marrow-derived dendritic cells, infection with human immunodeficiency virus, and immunopathology.Annu Rev Immunol. 1997;15:593-615. doi: 10.1146/annurev.immunol.15.1.593. Annu Rev Immunol. 1997. PMID: 9143701 Review.
-
Mechanisms promoting dendritic cell-mediated transmission of HIV.Mol Immunol. 2005 Feb;42(2):229-37. doi: 10.1016/j.molimm.2004.06.019. Mol Immunol. 2005. PMID: 15488610 Review.
Cited by
-
Quantitative expression and virus transmission analysis of DC-SIGN on monocyte-derived dendritic cells.J Virol. 2002 Sep;76(18):9135-42. doi: 10.1128/jvi.76.18.9135-9142.2002. J Virol. 2002. PMID: 12186897 Free PMC article.
-
Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells.J Virol. 2000 Jul;74(13):6087-95. doi: 10.1128/jvi.74.13.6087-6095.2000. J Virol. 2000. PMID: 10846092 Free PMC article.
-
Vaginal challenge with an SIV-based dual reporter system reveals that infection can occur throughout the upper and lower female reproductive tract.PLoS Pathog. 2014 Oct 9;10(10):e1004440. doi: 10.1371/journal.ppat.1004440. eCollection 2014 Oct. PLoS Pathog. 2014. PMID: 25299616 Free PMC article.
-
Protein-Protein Interaction between Surfactant Protein D and DC-SIGN via C-Type Lectin Domain Can Suppress HIV-1 Transfer.Front Immunol. 2017 Jul 31;8:834. doi: 10.3389/fimmu.2017.00834. eCollection 2017. Front Immunol. 2017. PMID: 28824609 Free PMC article.
-
Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus.J Virol. 2005 Jul;79(14):9217-27. doi: 10.1128/JVI.79.14.9217-9227.2005. J Virol. 2005. PMID: 15994816 Free PMC article.
References
-
- Allaerts W, Salomon B, Leenen P J M, Van Wijngaardt S, Jeucken P H M, Ruuls S, Klatzmann D, Drexhage H A. A population of interstitial cells in the anterior pituitary with a hematopoietic origin and a rapid turnover: a relationship with folliculo-stellate cells? J Neuroimmunol. 1997;78:184–197. - PubMed
-
- Balter M. HIV’s other immune-system targets: macrophages. Science. 1996;274:1464–1465. - PubMed
-
- Barrat-Boyes S M, Watkins S C, Finn O J. In vivo migration of dendritic cells differentiated in vitro. A chimpanzee model. J Immunol. 1997;158:4543–4547. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

