Carboxypeptidase D (gp180), a Golgi-resident protein, functions in the attachment and entry of avian hepatitis B viruses
- PMID: 9733850
- PMCID: PMC110147
- DOI: 10.1128/JVI.72.10.8098-8104.1998
Carboxypeptidase D (gp180), a Golgi-resident protein, functions in the attachment and entry of avian hepatitis B viruses
Abstract
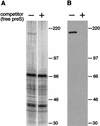
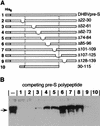
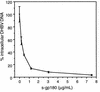
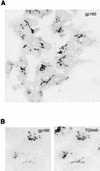
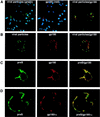
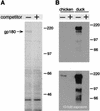
Carboxypeptidase D (gp180), one of many candidate receptors proposed for hepatitis B viruses (HBVs), was examined and found to be the actual cellular receptor for avian HBVs. This conclusion was based on the following observations: (i) gp180 was the only host protein that bound with high affinity to the pre-S ectodomain of the large duck hepatitis B virus (DHBV) envelope protein, which is known to be essential for virus infection; (ii) a pre-S subdomain which determines physical binding to gp180 was found to coincide with a domain functionally defined in infection competition experiments as a receptor binding domain; (iii) soluble gp180, lacking the membrane anchor, efficiently inhibited DHBV infection; (iv) efficient interspecies gp180-pre-S interaction was limited to the natural hosts of avian hepadnaviruses; and (v) expression of gp180 in a heterologous hepatoma cell line mediated cellular attachment and subsequent internalization of fluorescently labeled viral particles into vesicular structures. However, gp180 expression did not render transfected heterologous cells permissive for productive infection, suggesting that a species-specific coreceptor is required for fusion to complete viral entry. In contrast to the case for known virus receptors, gp180 was not detected on the hepatocyte cell surface but was found to be concentrated in the Golgi apparatus, from where it functions by cycling to and from the plasma membrane.
Figures
References
-
- Breiner, K. B., and H. Schaller. Unpublished data.
-
- DeMeyer S, Gong J Z, Suwandhi W, van Pelt J, Soumillon A, Yap S H. Organ and species specificity of hepatitis B virus (HBV) infection: a review of literature with a special reference to preferential attachment of HBV to human hepatocytes. J Viral Hepat. 1997;4:145–153. - PubMed
-
- Doms R, W, Peiper S C. Unwelcomed guests with master keys: how HIV uses chemokine receptors for cellular entry. Virology. 1997;235:179–190. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

