Development of a self-inactivating lentivirus vector
- PMID: 9733856
- PMCID: PMC110156
- DOI: 10.1128/JVI.72.10.8150-8157.1998
Development of a self-inactivating lentivirus vector
Abstract
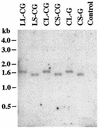
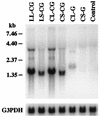
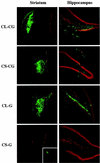
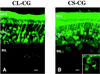
We have constructed a new series of lentivirus vectors based on human immunodeficiency virus type 1 (HIV-1) that can transduce nondividing cells. The U3 region of the 5' long terminal repeat (LTR) in vector constructs was replaced with the cytomegalovirus (CMV) promoter, resulting in Tat-independent transcription but still maintaining high levels of expression. A self-inactivating (SIN) vector was constructed by deleting 133 bp in the U3 region of the 3' LTR, including the TATA box and binding sites for transcription factors Sp1 and NF-kappaB. The deletion is transferred to the 5' LTR after reverse transcription and integration in infected cells, resulting in the transcriptional inactivation of the LTR in the proviruses. SIN viruses can be generated with no significant decreases in titer. Injection of viruses into the rat brain showed that a SIN vector containing the green fluorescent protein gene under the control of the internal CMV promoter transduced neurons as efficiently as a wild-type vector. Interestingly, a wild-type vector without an internal promoter also successfully transduced neurons in the brain, indicating that the HIV-1 LTR promoter is transcriptionally active in neurons even in the absence of Tat. Furthermore, injection of viruses into the subretinal space of the rat eye showed that wild-type vector transduced predominantly retinal pigment epithelium and photoreceptor cells, while SIN vector was able to transduce other types of retinal cells, including bipolar, Müller, horizontal, and amacrine cells. This finding suggests that the HIV-1 LTR can negatively influence the internal CMV promoter in some cell types. SIN HIV vectors should be safer for gene therapy, and they also have broader applicability as a means of high-level gene transfer and expression in nondividing cells.
Figures
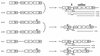
References
-
- Berkhout B, Gatignol A, Rabson A B, Jeang K-T. TAR-independent activation of the HIV-1 LTR: evidence that tat requires specific regions of the promoter. Cell. 1990;62:757–767. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

