Measles virus fusion protein is palmitoylated on transmembrane-intracytoplasmic cysteine residues which participate in cell fusion
- PMID: 9733862
- PMCID: PMC110167
- DOI: 10.1128/JVI.72.10.8198-8204.1998
Measles virus fusion protein is palmitoylated on transmembrane-intracytoplasmic cysteine residues which participate in cell fusion
Abstract
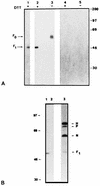
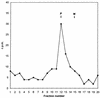
[3H]palmitic acid was metabolically incorporated into the viral fusion protein (F) of Edmonston or freshly isolated measles virus (MV) during infection of human lymphoid or Vero cells. The uncleaved precursor F0 and the F1 subunit from infected cells and extracellular virus were both labeled, indicating that palmitoylation can take place prior to F0 cleavage and that palmitoylated F protein was incorporated into virus particles. [3H]palmitic acid was released from F protein upon hydroxylamine or dithiothreitol treatment, indicating a thioester linkage. In cells transfected with the cloned MV F gene, in which the cysteines located in the intracytoplasmic and transmembrane domains (Cys 506, 518, 519, 520, and 524) were replaced by serine, a major reduction of [3H]palmitic acid incorporation was observed for F mutated at Cys 506 and, to a lesser extent, at Cys 518 and Cys 524. We also observed incorporation of [3H]palmitic acid in the F1 subunit of canine distemper virus F protein. Cell fusion induced by cotransfection of cells with MV F and H (hemagglutinin) genes was significantly reduced after replacement of Cys 506 or Cys 519 with serine in the MV F gene. Transfection with the F gene with a mutation for Cys 518 abolished cell fusion, although less mutant protein was detected on the cell surface. These results suggest that the F protein transmembrane domain cysteines 506 and 518 participate in structures involved in cell fusion, possibly mediated by palmitoylation.
Figures






References
-
- Bach R, Koningsberg W H, Nemerson Y. Human tissue contains thioester linked palmitate and stearate on the cytoplasmic half-cysteine. Biochemistry. 1988;27:4227–4231. - PubMed
-
- Bolt G, Blixenkrone-Moller M, Gottschalck E, Wishaupt R G A, Welsh M J, Earle J A P, Rima B K. Nucleotide and deduced amino acid sequences of the matrix (M) and fusion (F) protein genes of cetacean morbillivirus isolated from a porpoise and a dolphin. Virus Res. 1994;34:291–304. - PubMed
-
- Buckland R, Geralt C, Barker R, Wild T F. Fusion glycoprotein of measles virus: nucleotide sequence of the gene and comparison with other paramyxoviruses. J Gen Virol. 1987;68:1695–1703. - PubMed
-
- Buckland R, Malvoisin E, Beauverger P, Wild T F. A leucine zipper structure present in the measles virus fusion protein is not required for its tetramerization but is essential for fusion. J Gen Virol. 1992;73:1703–1707. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

