Efficient class II major histocompatibility complex presentation of endogenously synthesized hepatitis C virus core protein by Epstein-Barr virus-transformed B-lymphoblastoid cell lines to CD4(+) T cells
- PMID: 9733874
- PMCID: PMC110194
- DOI: 10.1128/JVI.72.10.8301-8308.1998
Efficient class II major histocompatibility complex presentation of endogenously synthesized hepatitis C virus core protein by Epstein-Barr virus-transformed B-lymphoblastoid cell lines to CD4(+) T cells
Abstract
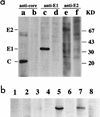
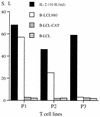
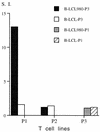
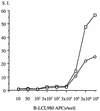
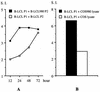
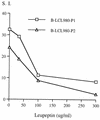
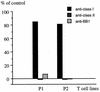
The induction of an efficient CD4(+) T-cell response against hepatitis C virus (HCV) is critical for control of the chronicity of HCV infection. The ability of HCV structural protein endogenously expressed in an antigen-presenting cell (APC) to be presented by class II major histocompatibility complex molecules to CD4(+) T cells was investigated by in vitro culture analyses using HCV core-specific T-cell lines and autologous Epstein-Barr virus-transformed B-lymphoblastoid cell lines (B-LCLs) expressing structural HCV antigens. The T- and B-cell lines were generated from peripheral blood mononuclear cells derived from HCV-infected patients. Expression and intracellular localization of core protein in transfected cells were determined by immunoblotting and immunofluorescence. By stimulation with autologous B-LCLs expressing viral antigens, strong T-cell proliferative responses were induced in two of three patients, while no substantial stimulatory effects were produced by B-LCLs expressing a control protein (chloramphenicol acetyltransferase) or by B-LCLs alone. The results showed that transfected B cells presented mainly endogenously synthesized core peptides. Presentation of secreted antigens from adjacent antigen-expressing cells was not enough to stimulate a core-specific T-cell response. Only weak T-cell proliferative responses were generated by stimulation with B-LCLs that had been pulsed beforehand with at least a 10-fold-higher amount of transfected COS cells in the form of cell lysate, suggesting that presentation of antigens released from dead cells in the B-LCL cultures had a minimal role. Titrating numbers of APCs, we showed that as few as 10(4) transfected B-LCL APCs were sufficient to stimulate T cells. This presentation pathway was found to be leupeptin sensitive, and it can be blocked by antibody to HLA class II (DR). In addition, expression of a costimulatory signal by B7/BB1 on B cells was essential for T-cell activation.
Figures

References
-
- Alter H J, Purcell R H, Shih J W, Melolder J C, Houghton M, Choo Q L. Detection of antibody to hepatitis C virus in prospectively followed transfusion recipients with acute and chronic non-A, non-B hepatitis. N Engl J Med. 1989;321:1494–1500. - PubMed
-
- Arichi, T., et al. Unpublished data.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

