Nucleoside analog 1592U89 and human immunodeficiency virus protease inhibitor 141W94 are synergistic in vitro
- PMID: 9736527
- PMCID: PMC105760
- DOI: 10.1128/AAC.42.9.2153
Nucleoside analog 1592U89 and human immunodeficiency virus protease inhibitor 141W94 are synergistic in vitro
Abstract
The use of combinations of anti-human immunodeficiency virus (anti-HIV) agents targeted to different molecular targets will most likely result in increased viral suppression and may also delay or prevent the emergence of resistant HIV strains. The purpose of the present study was to develop information on the in vitro anti-HIV activities of combinations of the reverse transcriptase inhibitor 1592U89 and the protease inhibitor 141W94 to help guide the choice of dosages in clinical trials. Triplicate in vitro dose-response matrices were prepared with MT-2 cells infected with HIV type 1 (HIV-1) strain IIIB. In order to account for the effects of protein binding, tissue culture medium with 10% fetal bovine serum was supplemented with the human serum proteins alpha1 acid glycoprotein (1 mg/ml) and albumin (40 mg/ml). The three-dimensional drug interaction surface for 1592U89 and 141W94 was constructed with the program MacSynergy II. As analyzed relative to a Bliss Independence null reference model, this combination was synergistic, with volumes of synergy exceeding 100 (99% confidence). Analysis of the data set with a fully parametric form of an equation for the quantitation of drug interaction developed by Greco et al. (W. R. Greco, G. Bravo, and J. C. Parsons, Pharmacol. Rev. 47:331-385, 1995) resulted in an interaction term statistically significantly greater than 0.0, indicating true synergy. Both methods concur that this combination is significantly synergistic. These data, with favorable findings from phase I/II trials for each drug alone, suggest that the combination of 1592U89 plus 141W94 should be further evaluated in clinical trials.
Figures
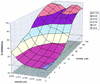

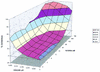


References
-
- Bilello J A, Bilello P A, Stellrecht K, Leonard J, Norrbeck D, Kempf D J, Robins T, Drusano G L. Human serum α1-acid glycoprotein reduces uptake, intracellular concentration, and antiviral activity of A-80987, an inhibitor of the human immunodeficiency virus type 1 protease. Antimicrob Agents Chemother. 1996;40:1491–1497. - PMC - PubMed
-
- D’Argenio D Z, Schumitzky A. User’s manual. University of Southern California: Biomedical Simulations Resource; 1990. ADAPT II: a program package for simulation identification and optimal experimental design. , Los Angeles.
-
- Greco W R, Bravo G, Parsons J C. The search for synergy: a critical review from a response surface perspective. Pharmacol Rev. 1995;47:331–385. - PubMed
-
- Molla A, Korneyeva M, Gao Q, Vasavasonda S, Schipper P J, Mo H M, Marowitz M, Chernyavskiy T, Niu P, Lyons N, Hsu A, Granneman G R, Ho D D, Boucher C A, Leonard J M, Norbeck D W, Kempf D J. Ordered accumulation of mutations in HIV protease confers resistance to ritonavir. Nat Med. 1996;2:270–276. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

