Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils
- PMID: 9736536
- PMCID: PMC105778
- DOI: 10.1128/AAC.42.9.2206
Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils
Abstract
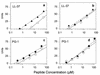
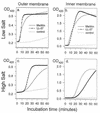
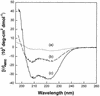
Human neutrophils contain two structurally distinct types of antimicrobial peptides, beta-sheet defensins (HNP-1 to HNP-4) and the alpha-helical peptide LL-37. We used radial diffusion assays and an improved National Committee for Clinical Laboratory Standards-type broth microdilution assay to compare the antimicrobial properties of LL-37, HNP-1, and protegrin (PG-1). Although generally less potent than PG-1, LL-37 showed considerable activity (MIC, <10 microgram/ml) against Pseudomonas aeruginosa, Salmonella typhimurium, Escherichia coli, Listeria monocytogenes, Staphylococcus epidermidis, Staphylococcus aureus, and vancomycin-resistant enterococci, even in media that contained 100 mM NaCl. Certain organisms (methicillin-resistant S. aureus, Proteus mirabilis, and Candida albicans) were resistant to LL-37 in media that contained 100 mM NaCl but were susceptible in low-salt media. Burkholderia cepacia was resistant to LL-37, PG-1, and HNP-1 in low- or high-salt media. LL-37 caused outer and inner membrane permeabilization of E. coli ML-35p. Chromogenic Limulus assays revealed that LL-37 bound to E. coli O111:B4 lipopolysaccharide (LPS) with a high affinity and that this binding showed positive cooperativity (Hill coefficient = 2.02). Circular dichroism spectrometry disclosed that LL-37 underwent conformational change in the presence of lipid A, transitioning from a random coil to an alpha-helical structure. The broad-spectrum antimicrobial properties of LL-37, its presence in neutrophils, and its inducibility in keratinocytes all suggest that this peptide and its precursor (hCAP-18) may protect skin and other tissues from bacterial intrusions and LPS-induced toxicity. The potent activity of LL-37 against P. aeruginosa, including mucoid and antibiotic-resistant strains, suggests that it or related molecules might have utility as topical bronchopulmonary microbicides in cystic fibrosis.
Figures




References
-
- Bruch M D, Dhingra M M, Gierasch L M. Side chain-backbone hydrogen bonding contributes to helix stability in peptides derived from an α-helical region of carboxypeptidase A. Proteins Struct Funct Genet. 1991;10:130–139. - PubMed
-
- Chen C, Brock R, Luh F, Chou P-J, Larrick J W, Huang R-F, Huang T-H. The solution structure of the active domain of CAP18—a lipopolysaccharide binding protein from rabbit leukocytes. FEBS Lett. 1995;370:46–52. - PubMed
-
- Chen Y H, Yang J T, Chau K H. Determination of the helix and β form of proteins in aqueous by circular dichroism. Biochemistry. 1974;13:3350–3359. - PubMed
-
- DeLeo F R, Quinn M T. Assembly of the phagocyte NADPH oxidase: molecular interaction of oxidase proteins. J Leukocyte Biol. 1996;60:677–691. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

