Effect of food on the bioavailability of stavudine in subjects with human immunodeficiency virus infection
- PMID: 9736552
- PMCID: PMC105822
- DOI: 10.1128/AAC.42.9.2295
Effect of food on the bioavailability of stavudine in subjects with human immunodeficiency virus infection
Abstract
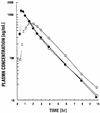
A randomized, three-way crossover study was carried out to determine the effects of food ingestion on the pharmacokinetics of stavudine (d4T). Fifteen subjects with human immunodeficiency virus (HIV) infection and CD4(+) cell counts of >/=200/microliter received 70 mg of d4T in a fasting state or 1 h before or 5 min after a standardized high-fat breakfast. A 7- to 15-day washout period was included between treatments. Blood and urine were collected before and for 10 h after dosing, and plasma and urine d4T concentrations were determined with a validated radioimmunoassay. Plasma drug concentration-time data were analyzed with a noncompartmental model. The mean maximum plasma drug concentration (Cmax) and the time to Cmax (Tmax) for administration of d4T after a meal were significantly lower and longer (P = 0.0001 for both measures) than those observed in the fasting state, although the area under the concentration-time curve from time zero to infinity (AUC0-infinity) was not significantly different. Neither of these parameters was significantly altered when d4T was taken 1 h before a meal. The bioavailability of d4T taken after a meal was 95% of that observed in the fasting state, and it was 97% when d4T was administered before a meal (P > 0.05 for both comparisons with the fasting state). The results of this study indicate that (i) ingestion of food does not affect the bioavailability of d4T and that patients with HIV infection can take it without regard to meals, and (ii) absorption is essentially complete within 1 h when d4T is administered in the fasted state.
Figures
References
-
- Blum, M. R., S. H. T. Liao, S. S. Good, and P. DeMiranda. 1988. Pharmacokinetics and bioavailability of zidovudine in humans. Am. J. Med. 85(Suppl. 2A):189–194. - PubMed
-
- Box G E P, Cox D R. Analysis of transformation. J R Stat Soc B. 1982;265:211–252.
-
- Browne M J, Mayer K H, Chafee S B D, Dudley M N, Posner M R, Steinberg S M, Graham K K, Geletko S M, Zinner S H, Denman S L, Dunkle L M, Kaul S, McLaren C, Skowron G, Kouttab N M, Kennedy T A, Weitberg A B, Curt G A. 2′,3′-Didehydro-3′-deoxythymidine (d4T) in patients with AIDS or AIDS-related complex: a phase I trial. J Infect Dis. 1993;167:21–29. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials