Identification of a survival-promoting peptide in medium conditioned by oxidatively stressed cell lines of nervous system origin
- PMID: 9736629
- PMCID: PMC6793258
- DOI: 10.1523/JNEUROSCI.18-18-07047.1998
Identification of a survival-promoting peptide in medium conditioned by oxidatively stressed cell lines of nervous system origin
Abstract
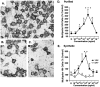
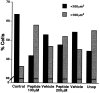
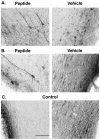
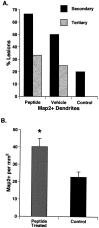
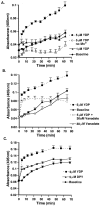
A survival-promoting peptide has been purified from medium conditioned by Y79 human retinoblastoma cells and a mouse hippocampal cell line (HN 33.1) exposed to H2O2. A 30 residue synthetic peptide was made on the basis of N-terminal sequences obtained during purification, and it was found to exhibit gel mobility and staining properties similar to the purified molecules. The peptide maintains cells and their processes in vitro for the HN 33.1 cell line treated with H2O2, and in vivo for cortical neurons after lesions of the cerebral cortex. It has weak homology with a fragment of a putative bacterial antigen and, like that molecule, binds IgG. The peptide also contains a motif reminiscent of a critical sequence in the catalytic region of calcineurin-type phosphatases; surprisingly, like several members of this family, the peptide catalyzes the hydrolysis of para-nitrophenylphosphate in the presence of Mn2+. Application of the peptide to one side of bilateral cerebral cortex lesions centered on area 2 in rats results in an increase in IgG immunoreactivity in the vicinity of the lesions 7 d after surgery. Microglia immunopositive for IgG and ED-1 are, however, dramatically reduced around the lesions in the treated hemisphere. Furthermore, pyramidal neurons that would normally shrink, die, or disintegrate were maintained, as determined by MAP2 immunocytochemistry and Nissl staining. These survival effects were often found in both hemispheres. The results suggest that this peptide operates by diffusion to regulate the immune response and thereby rescue neurons that would usually degenerate after cortical lesions. The phosphatase activity of this molecule also suggests the potential for direct neuron survival-promoting effects.
Figures




References
-
- Acharya HR, Dooley CM, Thoreson WB, Ahmad I. cDNA cloning and expression analysis of NeuroD mRNA in human retina. Biochem Biophys Res Commun. 1997;233:459–463. - PubMed
-
- Altschul SF, Boguski MS, Gish W, Wootton JC. Issues in searching molecular sequence databases. Nature Genet. 1994;6:119–129. - PubMed
-
- Beal MF. Aging, energy and oxidative stress in neurodegenerative diseases. Ann Neurol. 1995;38:357–366. - PubMed
-
- Bigot D, Hunt SP. Effect of excitatory amino acids on microtubule-associated proteins in cultured cortical and spinal neurones. Neurosci Lett. 1990;111:275–280. - PubMed
-
- Bigot D, Matus A, Hunt SP. Reorganization of the cytoskeleton in rat neurons following stimulation with excitatory amino acids in vitro. Eur J Neurosci. 1991;3:551–558. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
