Tau cleavage and dephosphorylation in cerebellar granule neurons undergoing apoptosis
- PMID: 9736630
- PMCID: PMC6793249
- DOI: 10.1523/JNEUROSCI.18-18-07061.1998
Tau cleavage and dephosphorylation in cerebellar granule neurons undergoing apoptosis
Abstract
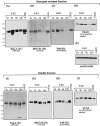
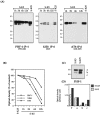
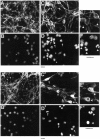
Cerebellar granule cells undergo apoptosis in culture after deprivation of potassium and serum. During this process we found that tau, a neuronal microtubule-associated protein that plays a key role in the maintenance of neuronal architecture, and the pathology of which correlates with intellectual decline in Alzheimer's disease, is cleaved. The final product of this cleavage is a soluble dephosphorylated tau fragment of 17 kDa that is unable to associate with microtubules and accumulates in the perikarya of dying cells. The appearance of this 17 kDa fragment is inhibited by both caspase and calpain inhibitors, suggesting that tau is an in vivo substrate for both of these proteases during apoptosis. Tau cleavage is correlated with disruption of the microtubule network, and experiments with colchicine and taxol show that this is likely to be a cause and not a consequence of tau cleavage. These data indicate that tau cleavage and change in phosphorylation are important early factors in the failure of the microtubule network that occurs during neuronal apoptosis. Furthermore, this study introduces new insights into the mechanism(s) that generate the truncated forms of tau present in Alzheimer's disease.
Figures
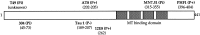



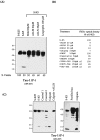


References
-
- Biernat J, Gustke N, Drewes G, Mandelkow EM, Mandelkow E. Phosphorylation at Ser 262 strongly reduces binding of tau to microtubules: distinction between PHF-like immunoreactivity and microtubule binding. Neuron. 1993;11:153–163. - PubMed
-
- Bonfoco E, Ceccatelli S, Manzo L, Nicotera L. Colchicine induces apoptosis in cerebellar granule cells. Exp Cell Res. 1995;218:189–200. - PubMed
-
- Brion JP, Hanger DP, Bruce MT, Couck AM, Flament-Durand J, Anderton BH. Tau in Alzheimer neurofibrillary tangles. N- and C-terminal regions are differentially associated with paired helical filaments and the location of a putative abnormal phosphorylation site. Biochem J. 1991;273:127–133. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
