Anion currents and predicted glutamate flux through a neuronal glutamate transporter
- PMID: 9736633
- PMCID: PMC6793262
- DOI: 10.1523/JNEUROSCI.18-18-07099.1998
Anion currents and predicted glutamate flux through a neuronal glutamate transporter
Abstract
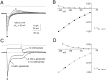
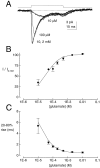
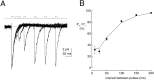
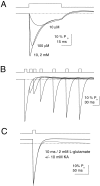
Kinetic properties of a native, neuronal glutamate transporter were studied by using rapid applications of glutamate to outside-out patches excised from Purkinje neurons. Pulses of glutamate activated anion currents associated with the transporter that were weakly antagonized by the transporter antagonist kainate. In addition, kainate blocked a resting anion conductance observed in the absence of glutamate. Transporter currents in response to glutamate concentration jumps under a variety of conditions were used to construct a cyclic kinetic model of the transporter. The model simulates both the anion conductance and the glutamate flux through the transporter, thereby permitting several predictions regarding the dynamics of glutamate transport at the synapse. For example, the concentration-dependent binding rate of glutamate to the transporter is high, similar to binding rates suggested for ligand-gated glutamate receptors. At saturating glutamate concentrations, transporters cycle at a steady-state rate of 13/sec. Transporters are predicted to have a high efficiency; once bound, a glutamate molecule is more likely to be transported than to unbind. Physiological concentrations of internal sodium and glutamate significantly slow net transport. Finally, a fixed proportion of anion and glutamate flux is expected over a wide range of circumstances, providing theoretical support for using net charge flux to estimate the amount and time course of glutamate transport.
Figures




Similar articles
-
Glial contribution to glutamate uptake at Schaffer collateral-commissural synapses in the hippocampus.J Neurosci. 1998 Oct 1;18(19):7709-16. doi: 10.1523/JNEUROSCI.18-19-07709.1998. J Neurosci. 1998. PMID: 9742141 Free PMC article.
-
Comparison of coupled and uncoupled currents during glutamate uptake by GLT-1 transporters.J Neurosci. 2002 Dec 1;22(23):10153-62. doi: 10.1523/JNEUROSCI.22-23-10153.2002. J Neurosci. 2002. PMID: 12451116 Free PMC article.
-
Postsynaptic response kinetics are controlled by a glutamate transporter at cone photoreceptors.J Neurophysiol. 1998 Jan;79(1):190-6. doi: 10.1152/jn.1998.79.1.190. J Neurophysiol. 1998. PMID: 9425190
-
[Role of glutamate transporters in excitatory synapses in cerebellar Purkinje cells].Brain Nerve. 2007 Jul;59(7):669-76. Brain Nerve. 2007. PMID: 17663137 Review. Japanese.
-
Electrogenic glutamate transporters in the CNS: molecular mechanism, pre-steady-state kinetics, and their impact on synaptic signaling.J Membr Biol. 2005 Jan;203(1):1-20. doi: 10.1007/s00232-004-0731-6. J Membr Biol. 2005. PMID: 15834685 Free PMC article. Review.
Cited by
-
Freshly isolated hippocampal CA1 astrocytes comprise two populations differing in glutamate transporter and AMPA receptor expression.J Neurosci. 2001 Oct 15;21(20):7901-8. doi: 10.1523/JNEUROSCI.21-20-07901.2001. J Neurosci. 2001. PMID: 11588163 Free PMC article.
-
Thallium ions can replace both sodium and potassium ions in the glutamate transporter excitatory amino acid carrier 1.Biochemistry. 2008 Dec 2;47(48):12923-30. doi: 10.1021/bi8017174. Biochemistry. 2008. PMID: 18986164 Free PMC article.
-
Regulation of astrocyte glutamine synthetase in epilepsy.Neurochem Int. 2013 Dec;63(7):670-81. doi: 10.1016/j.neuint.2013.06.008. Epub 2013 Jun 18. Neurochem Int. 2013. PMID: 23791709 Free PMC article. Review.
-
The role of perisynaptic glial sheaths in glutamate spillover and extracellular Ca(2+) depletion.Biophys J. 2001 Oct;81(4):1947-59. doi: 10.1016/S0006-3495(01)75846-8. Biophys J. 2001. PMID: 11566769 Free PMC article.
-
Synaptic activation of presynaptic glutamate transporter currents in nerve terminals.J Neurosci. 2003 Jun 15;23(12):4831-41. doi: 10.1523/JNEUROSCI.23-12-04831.2003. J Neurosci. 2003. PMID: 12832505 Free PMC article.
References
-
- Barbour B, Keller BU, Llano I, Marty A. Prolonged presence of glutamate during excitatory synaptic transmission to cerebellar Purkinje cells. Neuron. 1994;12:1331–1343. - PubMed
-
- Bergles DE, Jahr CE. Synaptic activation of glutamate transporters in hippocampal astrocytes. Neuron. 1997;19:1297–1308. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
