Functional redundancy of FMRFamide-related peptides at the Drosophila larval neuromuscular junction
- PMID: 9736637
- PMCID: PMC6793257
- DOI: 10.1523/JNEUROSCI.18-18-07138.1998
Functional redundancy of FMRFamide-related peptides at the Drosophila larval neuromuscular junction
Abstract
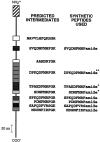
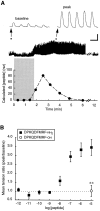
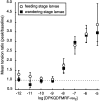
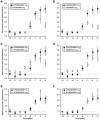
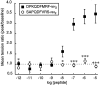
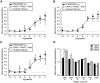
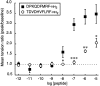
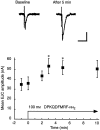
The Drosophila FMRFamide gene encodes multiple FMRFamide-related peptides. These peptides are expressed by neurosecretory cells and may be released into the blood to act as neurohormones. We analyzed the effects of eight of these peptides on nerve-stimulated contraction (twitch tension) of Drosophila larval body-wall muscles. Seven of the peptides strongly enhanced twitch tension, and one of the peptides was inactive. Their targets were distributed widely throughout the somatic musculature. The effects of one peptide, DPKQDFMRFamide, were unchanged after the onset of metamorphosis. The seven active peptides showed similar dose-response curves. Each had a threshold concentration near 1 nM, and the EC50 for each peptide was approximately 40 nM. At concentrations <0.1 microM, the responses to each of the seven excitatory peptides followed a time course that matched the fluctuations of the peptide concentration in the bath. At higher concentrations, twitch tension remained elevated for 5-10 min or more after wash-out of the peptide. When the peptides were presented as mixtures predicted by their stoichiometric ratios in the dFMRFamide propeptide, the effects were additive, and there were no detectable higher-order interactions among them. One peptide was tested and found to enhance synaptic transmission. At 0.1 microM, DPKQDFMRFamide increased the amplitude of the excitatory junctional current to 151% of baseline within 3 min. Together, these results indicate that the products of the Drosophila FMRFamide gene function as neurohormones to modulate the strength of contraction at the larval neuromuscular junction. In this role these seven peptides appear to be functionally redundant.
Figures


References
-
- Bainbridge SP, Bownes M. Staging the metamorphosis of Drosophila melanogaster. J Embryol Exp Morphol. 1981;66:57–80. - PubMed
-
- Bendena WG, Garside CS, Yu CG, Tobe SS. Allatostatins: diversity in structure and function of an insect neuropeptide family. Ann NY Acad Sci. 1997;814:53–66. - PubMed
-
- Benjamin PR, Burke JF. Alternative mRNA splicing of the FMRFamide gene and its role in neuropeptidergic signalling in a defined neural network. BioEssays. 1994;16:335–342. - PubMed
-
- Bleakman A, Bradbury AF, Darby NJ, Maruthainar K, Smyth DG. Processing reactions in the later stages of hormone activation. Biochimie. 1988;70:3–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
