Central P2X4 and P2X6 channel subunits coassemble into a novel heteromeric ATP receptor
- PMID: 9736638
- PMCID: PMC6793241
- DOI: 10.1523/JNEUROSCI.18-18-07152.1998
Central P2X4 and P2X6 channel subunits coassemble into a novel heteromeric ATP receptor
Abstract
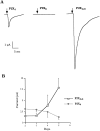
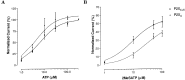
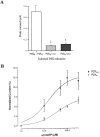
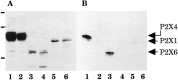
Ionotropic ATP receptors are widely expressed in mammalian CNS. Despite extensive functional characterization of neuronal homomeric P2X receptors in heterologous expression systems, the subunit composition of native central P2X ATP-gated channels remains to be elucidated. P2X4 and P2X6 are major central subunits with highly overlapping mRNA distribution at both regional and cellular levels. When expressed alone in Xenopus oocytes, P2X6 subunits do not assemble into surface receptors responsive to ATP applications. On the other hand, P2X4 subunits assemble into bona fide ATP-gated channels, slowly desensitizing and weakly sensitive to the partial agonist alpha,beta-methylene ATP and to noncompetitive antagonists suramin and pyridoxal-5-phosphate-6-azophenyl-2',4'-disulfonic acid. We demonstrate here that the coexpression of P2X4 and P2X6 subunits in Xenopus oocytes leads to the generation of a novel pharmacological phenotype of ionotropic ATP receptors. Heteromeric P2X4+6 receptors are activated by low-micromolar alpha, beta-methylene ATP (EC50 = 12 microM) and are blocked by suramin and by Reactive Blue 2, which has the property, at low concentrations, to potentiate homomeric P2X4 receptors. The assembly of P2X4 with P2X6 subunits results from subunit-dependent interactions, as shown by their specific copurification from HEK-293 cells transiently transfected with various epitope-tagged P2X channel subunits. Our data strongly suggest that the numerous cases of neuronal colocalizations of P2X4 and P2X6 subunits observed in mammalian CNS reflect the native expression of heteromeric P2X4+6 channels with unique functional properties.
Figures



References
-
- Bo X, Burnstock G. Distribution of [3H]α,β-methylene ATP binding sites in rat brain and spinal cord. NeuroReport. 1994;5:1601–1604. - PubMed
-
- Bo X, Zhang Y, Nassar M, Burnstock G, Schoepfer R. A P2X purinoceptor cDNA conferring a novel pharmacological profile. FEBS Lett. 1995;375:129–133. - PubMed
-
- Brake AJ, Wagenbach MJ, Julius D. New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature. 1994;371:519–523. - PubMed
-
- Buell G, Collo G, Rassendren F. P2X receptors: an emerging channel family. Eur J Neurosci. 1996;8:2221–2228. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
