Intracellular calcium and cell death during ischemia in neonatal rat white matter astrocytes in situ
- PMID: 9736645
- PMCID: PMC6793242
- DOI: 10.1523/JNEUROSCI.18-18-07232.1998
Intracellular calcium and cell death during ischemia in neonatal rat white matter astrocytes in situ
Abstract
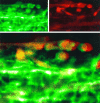
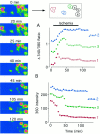
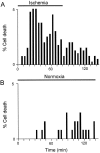
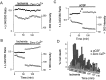
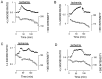
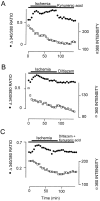
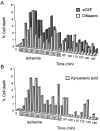
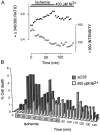
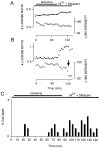
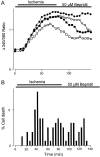
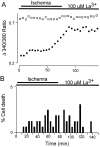
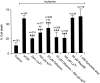
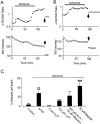
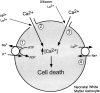
The major pathological correlate of cerebral palsy is ischemic injury of CNS white matter. Histological studies show early injury of glial cells and axons. To investigate glial cell injury, I monitored intracellular Ca2+ and cell viability in fura-2-loaded neonatal rat white matter glial cells during ischemia. Fura-2 fixation combined with immunohistochemistry revealed that fura-2-loaded cells were GFAP+/O4(-) and were therefore a population of neonatal white matter astrocytes. Significant ischemic Ca2+ influx was found, mediated by both L- and T-type voltage-gated Ca2+ channels. Ca2+ influx via T-type channels was the most important factor during the initial stage of ischemia and was associated with significant cell death within 10-20 min of the onset of ischemia. The Na+-Ca2+ exchanger acted to remove cytoplasmic Ca2+ throughout the ischemic and recovery periods. Neither the release of Ca2+ from intracellular stores nor influx via glutamate-gated channels contributed to the rise in intracellular Ca2+ during ischemia. Ischemic cell death was reduced significantly by removing extracellular Ca2+ or by blocking voltage-gated Ca2+ channels. The exclusively voltage-gated Ca2+ channel nature of the Ca2+ influx, the role played by T-type Ca2+ channels, the protective effect of the Na+-Ca2+ exchanger, and the lack of significant Ca2+ release from intracellular stores are features of ischemia that have not been reported in other CNS cell types.
Figures
Similar articles
-
In vitro ischemia promotes calcium influx and intracellular calcium release in hippocampal astrocytes.J Neurosci. 1996 Jan;16(1):71-81. doi: 10.1523/JNEUROSCI.16-01-00071.1996. J Neurosci. 1996. PMID: 8613811 Free PMC article.
-
Ionic mechanisms of anoxic injury in mammalian CNS white matter: role of Na+ channels and Na(+)-Ca2+ exchanger.J Neurosci. 1992 Feb;12(2):430-9. doi: 10.1523/JNEUROSCI.12-02-00430.1992. J Neurosci. 1992. PMID: 1311030 Free PMC article.
-
Potassium-dependent calcium influx in acutely isolated hippocampal astrocytes.Neuroscience. 1994 Jul;61(1):51-61. doi: 10.1016/0306-4522(94)90059-0. Neuroscience. 1994. PMID: 7969895
-
[Cerebral ischemia-hypoxia and biophysical mechanisms of neurodegeneration and neuroprotection effects].Fiziol Zh (1994). 2003;49(2):7-12. Fiziol Zh (1994). 2003. PMID: 12945108 Review. Ukrainian.
-
Action potential duration modulates calcium influx, Na(+)-Ca2+ exchange, and intracellular calcium release in rat ventricular myocytes.Ann N Y Acad Sci. 1996 Apr 15;779:417-29. doi: 10.1111/j.1749-6632.1996.tb44817.x. Ann N Y Acad Sci. 1996. PMID: 8659858 Review.
Cited by
-
Vascular endothelial growth factor increases the function of calcium-impermeable AMPA receptor GluA2 subunit in astrocytes via activation of protein kinase C signaling pathway.Glia. 2019 Jul;67(7):1344-1358. doi: 10.1002/glia.23609. Epub 2019 Mar 18. Glia. 2019. PMID: 30883902 Free PMC article.
-
Blockade of L-Type Ca2+ Channel Activity Alleviates Oligodendrocyte Pathology following Brain Injury in Male Rats.Curr Issues Mol Biol. 2023 May 2;45(5):3953-3964. doi: 10.3390/cimb45050252. Curr Issues Mol Biol. 2023. PMID: 37232721 Free PMC article.
-
mGluR5 protect astrocytes from ischemic damage in postnatal CNS white matter.Cell Calcium. 2015 Nov;58(5):423-30. doi: 10.1016/j.ceca.2015.06.010. Epub 2015 Jun 30. Cell Calcium. 2015. PMID: 26189008 Free PMC article.
-
Endoplasmic reticulum Ca2+ dysregulation and endoplasmic reticulum stress following in vitro neuronal ischemia: role of Na+-K+-Cl- cotransporter.J Neurochem. 2008 Aug;106(4):1563-76. doi: 10.1111/j.1471-4159.2008.05501.x. Epub 2008 Jun 28. J Neurochem. 2008. PMID: 18507737 Free PMC article.
-
GFP imaging of live astrocytes: regional differences in the effects of ischaemia upon astrocytes.J Anat. 2007 Jun;210(6):684-92. doi: 10.1111/j.1469-7580.2007.00731.x. J Anat. 2007. PMID: 17523937 Free PMC article. Review.
References
-
- Banker BQ, Larroche J-C. Periventricular leukomalacia of infancy: a form of neonatal anoxic encephalopathy. Arch Neurol. 1962;7:386–410. - PubMed
-
- Barres BA, Koroshetz WJ, Chun LL, Corey DP. Ion channel expression by white matter glia: the type-1 astrocyte. Neuron. 1990;5:527–544. - PubMed
-
- Bevensee MO, Schwiening CJ, Boron WF. Use of BCECF and propidium iodide to assess membrane integrity of acutely isolated CA1 neurons from rat hippocampus. J Neurosci Methods. 1995;58:61–75. - PubMed
-
- Butt AM, Ransom BR. Morphology of astrocytes and oligodendrocytes during development in the intact rat optic nerve. J Comp Neurol. 1993;338:141–158. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
