Regional selective neuronal degeneration after protein phosphatase inhibition in hippocampal slice cultures: evidence for a MAP kinase-dependent mechanism
- PMID: 9736650
- PMCID: PMC6793243
- DOI: 10.1523/JNEUROSCI.18-18-07296.1998
Regional selective neuronal degeneration after protein phosphatase inhibition in hippocampal slice cultures: evidence for a MAP kinase-dependent mechanism
Abstract
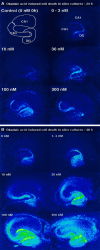
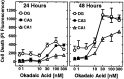
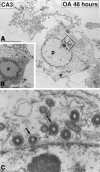
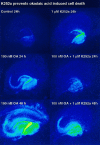
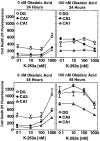
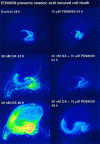
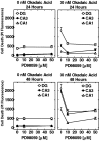
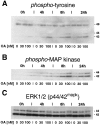
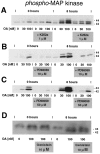
The regional selectivity and mechanisms underlying the toxicity of the serine/threonine protein phosphatase inhibitor okadaic acid (OA) were investigated in hippocampal slice cultures. Image analysis of propidium iodide-labeled cultures revealed that okadaic acid caused a dose- and time-dependent injury to hippocampal neurons. Pyramidal cells in the CA3 region and granule cells in the dentate gyrus were much more sensitive to okadaic acid than the pyramidal cells in the CA1 region. Electron microscopy revealed ultrastructural changes in the pyramidal cells that were not consistent with an apoptotic process. Treatment with okadaic acid led to a rapid and sustained tyrosine phosphorylation of the mitogen-activated protein kinases ERK1 and ERK2 (p44/42(mapk)). The phosphorylation was markedly reduced after treatment of the cultures with the microbial alkaloid K-252a (a nonselective protein kinase inhibitor) or the MAP kinase kinase (MEK1/2) inhibitor PD98059. K-252a and PD98059 also ameliorated the okadaic acid-induced cell death. Inhibitors of protein kinase C, Ca2+/calmodulin-dependent protein kinase II, or tyrosine kinase were ineffective. These results indicate that sustained activation of the MAP kinase pathway, as seen after e.g., ischemia, may selectively harm specific subsets of neurons. The susceptibility to MAP kinase activation of the CA3 pyramidal cells and dentate granule cells may provide insight into the observed relationship between cerebral ischemia and dementia in Alzheimer's disease.
Figures

References
-
- Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. - PubMed
-
- Ankarcrona M, Dypbukt JM, Orrenius S, Nicotera P. Calcineurin and mitochondrial function in glutamate-induced neuronal cell death. FEBS Lett. 1996;394:321–324. - PubMed
-
- Arendt T, Holzer M, Grossmann A, Zedlick D, Bruckner MK. Increased expression and subcellular translocation of the mitogen-activated protein kinase kinase and mitogen-activated protein kinase in Alzheimer’s disease. Neuroscience. 1995;68:5–18. - PubMed
-
- Benito A, Lerga A, Silva M, Leon J, Fernandez-Luna JL. Apoptosis of human myeloid leukemia cells induced by an inhibitor of protein phosphatases (okadaic acid) is prevented by Bcl-2 and Bcl-XL. Leukemia. 1997;11:940–944. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
