The survival-promoting effect of glial cell line-derived neurotrophic factor on axotomized corticospinal neurons in vivo is mediated by an endogenous brain-derived neurotrophic factor mechanism
- PMID: 9736655
- PMCID: PMC6793255
- DOI: 10.1523/JNEUROSCI.18-18-07351.1998
The survival-promoting effect of glial cell line-derived neurotrophic factor on axotomized corticospinal neurons in vivo is mediated by an endogenous brain-derived neurotrophic factor mechanism
Abstract
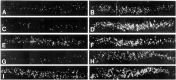
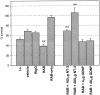
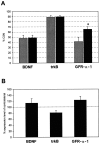
Autocrine trophic functions of brain-derived neurotrophic factor (BDNF) have been proposed for many central neurons because this neurotrophin displays striking colocalization with its receptor trkB within the CNS. In the cortex, the distribution patterns of BDNF and trkB expression are almost identical. Corticospinal neurons (CSNs) are a major cortical long-distance projecting system. They are localized in layer V of the somatosensory cortex, and their axons project into the spinal cord where they contribute to the innervation of spinal motoneurons. We have shown recently that adult CSNs express trkB mRNA and are rescued from axotomy-induced death by BDNF treatment. Half of the axotomized CSNs survived without BDNF infusions. These findings raise the possibility that endogenous cortical BDNF is involved in the trophic support of this neuronal population. To test the hypothesis that endogenous cortical BDNF promotes survival of adult CSNs, we infused the BDNF-neutralizing affinity-purified antibody RAB to axotomized and unlesioned CSNs for 7 d. This treatment resulted in increased death of axotomized CSNs. Survival of unlesioned CSNs was not affected by RAB treatment. In situ hybridizations for BDNF and trkB mRNA revealed that virtually all CSNs express trkB, whereas only half of them express BDNF. Thus, autocrine/paracrine mechanisms are likely to contribute to the endogenous BDNF protection of axotomized CSNs. We have demonstrated previously that, in addition to BDNF, glial cell line-derived neurotrophic factor (GDNF) and neurotrophin 3 (NT-3) also rescue CSNs from axotomy-induced death. We now show that the rescuing by GDNF requires the presence of endogenous cortical BDNF, implicating a central role of this neurotrophin in the trophic support of axotomized CSNs and a trophic cross-talk between BDNF and GDNF regarding the maintenance of lesioned CSNs. In contrast, NT-3 promotes survival of axotomized CSNs even when endogenous cortical BDNF is neutralized by RAB, indicating a potential of compensatory mechanisms for the trophic support of CSNs.
Figures

References
-
- Acheson A, Lindsay RM. Non-target-derived roles of the neurotrophins. Philos Trans R Soc Lond B Biol Sci. 1996;351:417–422. - PubMed
-
- Acheson A, Conover JC, Fandl JP, DeChiara TM, Russell M, Thadani A, Squinto SP, Yancopoulos GD, Lindsay RM. A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature. 1995;374:450–453. - PubMed
-
- Aguayo AJ, Rasminsky M, Bray GM, Carbonetto S, McKerracher L, Villegas Perez MP, Vidal Sanz M, Carter DA. Degenerative and regenerative responses of injured neurons in the central nervous system of adult mammals. Philos Trans R Soc Lond B Biol Sci. 1991;331:337–343. - PubMed
-
- Altar CA, Siuciak JA, Wright P, Ip NY, Lindsay RM, Wiegand SJ. In situ hybridization of trkB and trkC receptor mRNA in rat forebrain and association with high-affinity binding of [125I]BDNF, [125I]NT-4/5 and [125I]NT-3. Eur J Neurosci. 1994;6:1389–1405. - PubMed
-
- Barbacid M. The Trk family of neurotrophin receptors. J Neurobiol. 1994;25:1386–1403. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
