The natural polyamine spermine functions directly as a free radical scavenger
- PMID: 9736703
- PMCID: PMC21609
- DOI: 10.1073/pnas.95.19.11140
The natural polyamine spermine functions directly as a free radical scavenger
Abstract
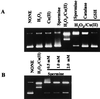
The polyamines are small organic cations that are absolutely required for eukaryotic cell growth. Although their growth requirements are well established, the molecular functions of the polyamines are ill-defined. Oxidative damage to DNA by reactive oxygen species is a continual problem that cells must guard against to survive. The polyamine spermine, which is normally found in millimolar concentrations in the nucleus, is shown here to function directly as a free radical scavenger, and adducts formed as a result of this function are identified. These data suggest that spermine is a major natural intracellular compound capable of protecting DNA from free radical attack.
Figures



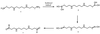
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

