Interaction of HIV-1 Nef with the cellular dileucine-based sorting pathway is required for CD4 down-regulation and optimal viral infectivity
- PMID: 9736718
- PMCID: PMC21624
- DOI: 10.1073/pnas.95.19.11229
Interaction of HIV-1 Nef with the cellular dileucine-based sorting pathway is required for CD4 down-regulation and optimal viral infectivity
Abstract
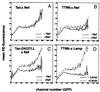
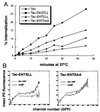
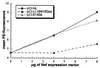
The HIV-1 Nef protein is important for pathogenesis, enhances viral infectivity, and regulates the sorting of at least two cellular transmembrane proteins, CD4 and major histocompatibility complex (MHC) class I. Although several lines of evidence support the hypothesis that the Nef protein interacts directly with the cellular protein sorting machinery, the sorting signal in HIV-1 Nef has not been identified. By using a competition assay that functionally discriminates between dileucine-based and tyrosine-based sorting signals, we have categorized the motif through which Nef interacts with the sorting machinery as dileucine-based. Inspection of diverse Nef proteins from HIV-1, HIV-2, and simian immunodeficiency virus revealed a well-conserved sequence in the central region of the C-terminal, solvent-exposed loop of Nef (E/DXXXLphi) that conforms to the consensus sequence of the dileucine-based sorting motifs found in cellular transmembrane proteins. This sequence in NefNL4-3, ENTSLL, functioned as an endocytosis signal when appended to the cytoplasmic tail of a heterologous protein. The leucine residues in this motif were required for the interaction of full-length Nef with the dileucine-based sorting pathway and were required for Nef-mediated down-regulation of CD4. These leucine residues were also required for optimal viral infectivity. These data indicate that a dileucine-based sorting signal in Nef is utilized to address the cellular sorting machinery. The data also suggest that an influence on the distribution of cellular transmembrane proteins may mechanistically unite two previously distinct properties of Nef: down-regulation of CD4 and enhancement of viral infectivity.
Figures

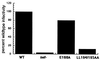
Similar articles
-
A dileucine motif in HIV-1 Nef is essential for sorting into clathrin-coated pits and for downregulation of CD4.Curr Biol. 1998 Nov 5;8(22):1239-42. doi: 10.1016/s0960-9822(07)00518-0. Curr Biol. 1998. PMID: 9811611
-
The dileucine-based sorting motif in HIV-1 Nef is not required for down-regulation of class I MHC.Virology. 1999 Jun 5;258(2):203-7. doi: 10.1006/viro.1999.9736. Virology. 1999. PMID: 10366557
-
A dileucine motif in HIV-1 Nef acts as an internalization signal for CD4 downregulation and binds the AP-1 clathrin adaptor.Curr Biol. 1998 Nov 5;8(22):1235-8. doi: 10.1016/s0960-9822(07)00517-9. Curr Biol. 1998. PMID: 9811606
-
[The HIV nef and the Kaposi-sarcoma-associated virus K3/K5 proteins: "parasites"of the endocytosis pathway].Med Sci (Paris). 2003 Jan;19(1):100-6. doi: 10.1051/medsci/2003191100. Med Sci (Paris). 2003. PMID: 12836198 Review. French.
-
HIV-1 Nef: a critical factor in viral-induced pathogenesis.Adv Pharmacol. 2000;48:299-343. doi: 10.1016/s1054-3589(00)48010-5. Adv Pharmacol. 2000. PMID: 10987095 Review. No abstract available.
Cited by
-
Nef decreases HIV-1 sensitivity to neutralizing antibodies that target the membrane-proximal external region of TMgp41.PLoS Pathog. 2011 Dec;7(12):e1002442. doi: 10.1371/journal.ppat.1002442. Epub 2011 Dec 15. PLoS Pathog. 2011. PMID: 22194689 Free PMC article.
-
S2 from equine infectious anemia virus is an infectivity factor which counteracts the retroviral inhibitors SERINC5 and SERINC3.Proc Natl Acad Sci U S A. 2016 Nov 15;113(46):13197-13202. doi: 10.1073/pnas.1612044113. Epub 2016 Nov 1. Proc Natl Acad Sci U S A. 2016. PMID: 27803322 Free PMC article.
-
Disrupting surfaces of nef required for downregulation of CD4 and for enhancement of virion infectivity attenuates simian immunodeficiency virus replication in vivo.J Virol. 2000 Nov;74(21):9836-44. doi: 10.1128/jvi.74.21.9836-9844.2000. J Virol. 2000. PMID: 11024110 Free PMC article.
-
Nef-induced major histocompatibility complex class I down-regulation is functionally dissociated from its virion incorporation, enhancement of viral infectivity, and CD4 down-regulation.J Virol. 2000 Mar;74(6):2907-12. doi: 10.1128/jvi.74.6.2907-2912.2000. J Virol. 2000. PMID: 10684310 Free PMC article.
-
The activity of Nef on HIV-1 infectivity.Front Microbiol. 2014 May 20;5:232. doi: 10.3389/fmicb.2014.00232. eCollection 2014. Front Microbiol. 2014. PMID: 24904546 Free PMC article. Review.
References
-
- Kestler H W, III, Ringler D J, Mori K, Panicali D L, Sehgal P K, Daniel M D, Desrosiers R C. Cell. 1991;65:651–662. - PubMed
-
- Deacon N J, Tsykin A, Solomon A, Smith K, Ludford-Menting M, Hooker D J, McPhee D A, Greenway A L, Ellett A, Chatfield C, et al. Science. 1995;270:988–991. - PubMed
-
- de Ronde A, Klaver B, Keulen W, Smit L, Goudsmit J. Virology. 1992;187:391–395. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

