A dysfunctional desmin mutation in a patient with severe generalized myopathy
- PMID: 9736733
- PMCID: PMC21639
- DOI: 10.1073/pnas.95.19.11312
A dysfunctional desmin mutation in a patient with severe generalized myopathy
Abstract
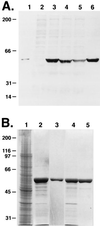
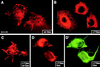
Mice lacking desmin produce muscle fibers with Z disks and normal sarcomeric organization. However, the muscles are mechanically fragile and degenerate upon repeated contractions. We report here a human patient with severe generalized myopathy and aberrant intrasarcoplasmic accumulation of desmin intermediate filaments. Muscle tissue from this patient lacks the wild-type desmin allele and has a desmin gene mutation encoding a 7-aa deletion within the coiled-coil segment of the protein. We show that recombinant desmin harboring this deletion cannot form proper desmin intermediate filament networks in cultured cells, nor is it able to assemble into 10-nm filaments in vitro. These findings provide direct evidence that a mutation in desmin can cause human myopathies.
Figures




References
-
- Li Z, Marchand P, Humbert J, Babinet C, Paulin D. Development (Cambridge, UK) 1993;117:947–959. - PubMed
-
- Gard D L, Lazarides E. Cell. 1980;19:263–275. - PubMed
-
- Lazarides E, Granger B L, Gard D L, O’Connor C M, Breckler J, Price M, Danto S I. Cold Spring Harbor Symp Quant Biol. 1982;46:351–378. - PubMed
-
- Fuchs E, Cleveland D. Science. 1998;279:514–519. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

