Molecular markers for cell types of the inner ear and candidate genes for hearing disorders
- PMID: 9736748
- PMCID: PMC21654
- DOI: 10.1073/pnas.95.19.11400
Molecular markers for cell types of the inner ear and candidate genes for hearing disorders
Abstract
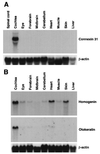
To identify genes expressed in the vertebrate inner ear, we have established an assay that allows rapid analysis of the differential expression pattern of mRNAs derived from an auditory epithelium-specific cDNA library. We performed subtractive hybridization to create an enriched probe, which then was used to screen the cDNA library. After digoxigenin-labeled antisense cRNAs had been transcribed from hybridization-positive clones, we conducted in situ hybridization on slides bearing cryosections of late embryonic chicken heads, bodies, and cochleae. One hundred and twenty of the 196 clones analyzed encode 12 proteins whose mRNAs are specifically or highly expressed in the chicken's inner ear; the remainder encode proteins that occur more widely. We identified proteins that have been described previously as expressed in the inner ear, such as beta-tectorin, calbindin, and type II collagen. A second group of proteins abundant in the inner ear includes five additional types of collagens. A third group, including Coch-5B2 and an ear-specific connexin, comprises proteins whose human equivalents are candidates to account for hearing disorders. This group also includes proteins expressed in two unique cell types of the inner ear, homogene cells and cells of the tegmentum vasculosum.
Figures


Similar articles
-
Distribution of beta-tectorin mRNA in the early posthatch and developing avian inner ear.Hear Res. 1996 Jul;96(1-2):167-78. doi: 10.1016/0378-5955(96)00045-7. Hear Res. 1996. PMID: 8817316
-
Tectorin mRNA expression is spatially and temporally restricted during mouse inner ear development.J Comp Neurol. 1999 Mar 8;405(2):271-80. J Comp Neurol. 1999. PMID: 10023815
-
Molecular cloning of chick beta-tectorin, an extracellular matrix molecule of the inner ear.J Cell Biol. 1995 Apr;129(2):535-47. doi: 10.1083/jcb.129.2.535. J Cell Biol. 1995. PMID: 7721949 Free PMC article.
-
An introduction to the genetics of normal and defective hearing.Ann N Y Acad Sci. 1997 Dec 29;830:361-74. doi: 10.1111/j.1749-6632.1997.tb51908.x. Ann N Y Acad Sci. 1997. PMID: 9616696 Review.
-
[Genetic type of progressive hearing loss].Acta Otorhinolaryngol Ital. 1998 Aug;18(4 Suppl 59):21-7. Acta Otorhinolaryngol Ital. 1998. PMID: 10205929 Review. Italian.
Cited by
-
Deafness-Associated ADGRV1 Mutation Impairs USH2A Stability through Improper Phosphorylation of WHRN and WDSUB1 Recruitment.Adv Sci (Weinh). 2023 Jun;10(16):e2205993. doi: 10.1002/advs.202205993. Epub 2023 Apr 17. Adv Sci (Weinh). 2023. PMID: 37066759 Free PMC article.
-
Analysis of gelsolin expression pattern in developing chicken embryo reveals high GSN expression level in tissues of neural crest origin.Brain Struct Funct. 2016 Jan;221(1):515-34. doi: 10.1007/s00429-014-0923-5. Epub 2014 Oct 29. Brain Struct Funct. 2016. PMID: 25352156 Free PMC article.
-
Pinpointing the expression of piRNAs and function of the PIWI protein subfamily during spermatogenesis in the mouse.Dev Biol. 2011 Jul 15;355(2):215-26. doi: 10.1016/j.ydbio.2011.04.021. Epub 2011 Apr 22. Dev Biol. 2011. PMID: 21539824 Free PMC article.
-
Plasma Membrane Targeting of Protocadherin 15 Is Regulated by the Golgi-Associated Chaperone Protein PIST.Neural Plast. 2016;2016:8580675. doi: 10.1155/2016/8580675. Epub 2016 Oct 27. Neural Plast. 2016. PMID: 27867666 Free PMC article.
-
Angulin proteins ILDR1 and ILDR2 regulate alternative pre-mRNA splicing through binding to splicing factors TRA2A, TRA2B, or SRSF1.Sci Rep. 2017 Aug 7;7(1):7466. doi: 10.1038/s41598-017-07530-z. Sci Rep. 2017. PMID: 28785060 Free PMC article.
References
-
- Schweinfest C W, Henderson K W, Gu I R, Kottaridis S T, Besbeas S, Panotopoulou E, Papas T S. Genet Ann Technol Appl. 1990;7:64–70. - PubMed
-
- Robertson N G, Khetarpal U, Gutierrez-Espeleta G A, Bieber F R, Morton C C. Genomics. 1994;23:42–50. - PubMed
-
- Bonaldo M F, Lennon G, Soares M B. Genome Res. 1996;6:791–806. - PubMed
-
- Steel K P, Brown S D M. Trends Genet. 1994;10:428–435. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

