Expression, characterization, processing and immunogenicity of an insulin-dependent diabetes mellitus autoantigen, IA-2, in Sf-9 cells
- PMID: 9737664
- PMCID: PMC1905060
- DOI: 10.1046/j.1365-2249.1998.00676.x
Expression, characterization, processing and immunogenicity of an insulin-dependent diabetes mellitus autoantigen, IA-2, in Sf-9 cells
Abstract
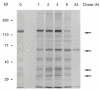
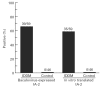
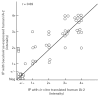
Autoantibodies to a 64-kD protein and a 40-kD tryptic fragment from pancreatic islets have been detected at high frequency in the sera of patients with insulin-dependent diabetes mellitus (IDDM). IA-2, a newly isolated transmembrane protein tyrosine phosphatase, is a major islet cell autoantigen in IDDM and the precursor of a 40-kD tryptic fragment. To express large quantities of recombinant IA-2 protein and analyse post-translational modifications we expressed full-length human IA-2 in baculovirus-infected Sf-9 cells. IA-2 expression was analysed by Western blot and by immunoprecipitation of 35S-methionine-radiolabelled proteins with rabbit antisera or IDDM sera. A 120-kD IA-2 protein was detected during the early, but not the late, phase of the infection. Pulse-chase experiments showed that the 120-kD protein was processed into fragments of 64 kD and smaller fragments of approximately 50 kD, 38 kD and 32 kD. The 64-kD fragment appeared as a doublet. Tunicamycin and PNGase F treatment down-shifted the 120-kD protein and the 64-kD doublet into lower molecular weight bands, suggesting that both were glycosylated. Trypsin treatment converted the 120-kD protein and the 64-kD doublet into a 40-kD fragment. Baculovirus-expressed IA-2 was as sensitive or slightly more sensitive than in vitro translated IA-2 in detecting autoantibodies to IA-2: 66% of sera from newly diagnosed IDDM patients reacted with baculovirus-expressed IA-2 compared with 59% of the same sera which reacted with in vitro translated IA-2. It is concluded that baculovirus-expressed IA-2 is a good source of autoantigen and that a number of lower molecular weight fragments with which IDDM autoantibodies react are derived from the 120-kD full-length IA-2 molecule.
Figures






References
-
- Atkinson MA, Maclaren NK. The pathogenesis of insulin-dependent diabetes mellitus. N Engl J Med. 1994;331:1427–36. - PubMed
-
- Bonifacio E, Bingley PJ, Shattock M, Dean BM, Dunger D, Gale EAM, Bottazzo GF. Quantification of islet-cell antibodies and prediction of insulin-dependent diabetes. Lancet. 1990;335:147–9. - PubMed
-
- Christie MR, Tun RY, Lo SS, et al. Antibodies to GAD and tryptic fragments of islet 64K antigen as distinct markers for development of IDDM. Studies with identical twins. Diabetes. 1992;41:782–7. - PubMed
-
- Christie MR, Genovese S, Cassidy D, Bosi E, Brown TJ, Lai M, Bonifacio E, Bottazzo GF. Antibodies to islet 37k antigen, but not to glutamate decarboxylase, discriminate rapid progression to IDDM in endocrine autoimmunity. Diabetes. 1994;43:1254–9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

