The murine buccal mucosa is an inductive site for priming class I-restricted CD8+ effector T cells in vivo
- PMID: 9737667
- PMCID: PMC1905068
- DOI: 10.1046/j.1365-2249.1998.00671.x
The murine buccal mucosa is an inductive site for priming class I-restricted CD8+ effector T cells in vivo
Abstract
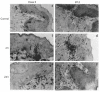
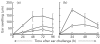
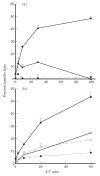
The present study shows that Langerhans cells of the buccal mucosa and the skin share a similar phenotype, including in situ expression of MHC class II, the mannose receptor DEC-205 and CD11c, and absence of the costimulatory molecules B7.1, B7.2 and CD40 as well as Fas. Application of 2,4-dinitrofluorobenzene (DNFB) onto the buccal mucosa is associated with a rapid migration of dendritic cells (DC) to the epithelium and induction of B7.2 expression on some DC. Buccal sensitization with DNFB elicited a specific contact sensitivity (CS) in response to skin challenge, mediated by class I-restricted CD8+ effector T cells and down-regulated by class II-restricted CD4+ T cells, demonstrated by the lack of priming of class I-deficient mice and the enhanced response of class II-deficient mice, respectively. CS induced by buccal immunization is associated with priming of class I-restricted CD8+ effector T cells endowed with hapten-specific cytotoxic activity. Thus, the buccal epithelium is an inductive site, equivalent to the epidermis, for the generation of CS independent of CD4 help, and of cytotoxic T lymphocyte (CTL) responses mediated by class I-restricted CD8+ T cells. We propose that immunization through the buccal mucosa, which allows antigen presentation by epithelial DC efficient for priming systemic class I-restricted CD8+ CTL, may be a valuable approach for single-dose mucosal vaccination with subunit vaccines.
Figures

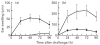

References
-
- Butcher EC. The regulation of lymphocyte traffic. Curr Top Microbiol Immunol. 1986;128:85–95. - PubMed
-
- Gowans JL, Knight EJ. The route of re-circulation of lymphocytes in the rat. Proc R Soc Lond B. 1964;159:257–3. - PubMed
-
- McDermott MR, Bienenstock J. Evidence for a common mucosal immunological system. I. Migration of B immunoblasts into intestinal, respiratory, and genital tissues. J Immunol. 1979;122:1892–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

