A variety of cytokines and immunologically relevant surface molecules are expressed by normal human skeletal muscle cells under proinflammatory stimuli
- PMID: 9737670
- PMCID: PMC1905062
- DOI: 10.1046/j.1365-2249.1998.00664.x
A variety of cytokines and immunologically relevant surface molecules are expressed by normal human skeletal muscle cells under proinflammatory stimuli
Abstract
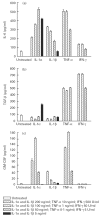
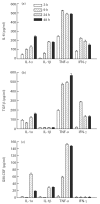
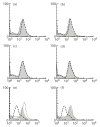
Muscle is an attractive target for gene therapy and for immunization with DNA vaccines and is also the target of immunological injury in myositis. It is important therefore to understand the immunologic capabilities of muscle cells themselves. In this study, we show that proinflammatory stimuli induce the expression of other cytokines such as IL-6, transforming growth factor-beta (TGF-beta), and granulocyte-macrophage colony-stimulating factor (GM-CSF) by muscle cells themselves, as well as the up-regulation of human leucocyte antigen (HLA) class I, class II and intercellular adhesion molecule-1 (ICAM-1). Thus, muscle cells have an inherent ability to express and respond to a variety of cytokines and chemokines. The levels of HLA class I, class II and ICAM-1 in inflamed muscle may be affected by the secreted products of the stimulation.
Figures

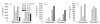

References
-
- Streilein JW. Peripheral tolerance induction: lessons from immune privileged sites and tissues. Transplant Proc. 1996;28:2066–70. - PubMed
-
- Law PK, Goodwin TG, Fang Q, et al. Human gene therapy with myoblast transfer. Transplant Proc. 1997;29:2234–7. - PubMed
-
- Kim JJ, Bagarazzi ML, Trivedi N, et al. Engineering of in vivo immune responses to DNA immunization via codelivery of costimulatory molecule genes. Nat Biotechnol. 1997;15:641–6. - PubMed
-
- Plotz PH, Miller FW. Animal models of myositis. Mt Sinai J Med (NY) 1988;55:501–5. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

