Ultrasensitive reverse transcription-PCR assay for quantitation of human immunodeficiency virus type 1 RNA in plasma
- PMID: 9738051
- PMCID: PMC105095
- DOI: 10.1128/JCM.36.10.2964-2969.1998
Ultrasensitive reverse transcription-PCR assay for quantitation of human immunodeficiency virus type 1 RNA in plasma
Abstract
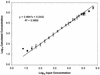
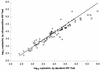
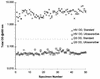
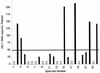
With the recent introduction of combination therapy, human immunodeficiency virus type 1 (HIV-1) RNA levels in plasma have been dramatically reduced, frequently to below the limit of quantitation (400 copies/ml of plasma) of the AMPLICOR HIV-1 MONITOR Test (Roche Diagnostic Systems). To achieve enhanced sensitivity of the AMPLICOR HIV-1 MONITOR Test, a modified specimen preparation procedure that allows input of RNA from 10-fold more plasma per amplification reaction was developed. This "ultrasensitive" method allows the accurate quantitation of plasma HIV-1 RNA levels as low as 50 copies/ml. A precision study yielded average within-run and between-run coefficients of variation (CV) of 24.8 and 9.6%, respectively. A multicenter reproducibility study demonstrated that the laboratory-to-laboratory reproducibility of this assay is good, with an average CV of 32%. The linear range of this test is between 50 and 50,000 copies/ml of plasma. RNA concentrations measured by the ultrasensitive and standard HIV-1 MONITOR tests exhibited good agreement within the shared linear range of the two methods. The two measurements were within a factor of 2 for 91% of the specimens tested, with the concentration measured by the ultrasensitive method being only slightly lower (median, 22% lower). Preliminary studies suggest that this assay will prove to be useful for predicting the stability of viral suppression in patients whose RNA levels drop below 400 copies/ml in response to highly active antiretroviral therapy.
Figures
References
-
- BHIVA Guidelines Co-ordinating Committee. British HIV Association guidelines for antiretroviral treatment of HIV seropositive individuals. Lancet. 1997;349:1086–1092. - PubMed
-
- Carpenter C C, Fischl M A, Hammer S M, Hirsch M S, Jacobsen D M, Katzenstein D A, Montaner J S, Richman D D, Saag M S, Schooley R T, Thompson M A, Vella S, Yeni P G, Volberding P A for the International AIDS Society-USA. Antiretroviral therapy for HIV infection in 1996. Recommendations of an international panel. JAMA. 1996;276:146–154. - PubMed
-
- Centers for Disease Control and Prevention. Public Health Service task force recommendations for the use of antiretroviral drugs in pregnant women infected with HIV-1 for maternal health and for reducing perinatal HIV-1 transmission in the United States. Morbid Mortal Weekly Rep. 1998;47(No. RR-2):1–39. - PubMed
-
- Centers for Disease Control and Prevention. Guidelines for the use of antiretroviral agents in pediatric HIV infection. Morbid Mortal Weekly Rep. 1998;47(No. RR-4):1–51. - PubMed
-
- Eron J J, Benoit S L, Jemsek J, MacArthur R D, Santana J, Quinn J B, Kuritzkes D R, Fallon M A, Rubin and the North American HIV Working Party M. Treatment with lamivudine, zidovudine, or both in HIV-positive patients with 200 to 500 CD4+ cells per cubic millimeter. N Engl J Med. 1995;333:1662–1669. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

