Novel regulatory factors interacting with the promoter of the gene encoding the mRNA cap binding protein (eIF4E) and their function in growth regulation
- PMID: 9742079
- PMCID: PMC109148
- DOI: 10.1128/MCB.18.10.5621
Novel regulatory factors interacting with the promoter of the gene encoding the mRNA cap binding protein (eIF4E) and their function in growth regulation
Abstract
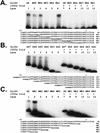
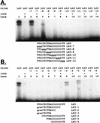
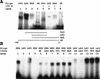
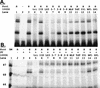
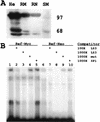
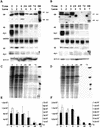
Regulation of the mRNA cap binding protein (eIF4E) is critical to the control of cellular proliferation since this protein is the rate-limiting factor in translation initiation and transforms fibroblasts and since eIF4E mutants arrest budding yeast in the G1 phase of the cell cycle (cdc33). We previously demonstrated regulation of eIF4E by altered transcription of its mRNA in serum-stimulated fibroblasts and in response to c-myc. To identify additional factors regulating eIF4E transcription, we used linker-scanning constructs to characterize sites in the promoter of the eIF4E gene required for its expression. Promoter activity was dependent on sites at -5, -25, -45, and -75; the site at -75 included a previously described myc box. Electrophoretic mobility shift assays identified DNA-protein interactions at -25 and revealed a binding site (TTACCCCCCCTT) that is unique to the eIF4E promoter. Proteins of 68 and 97 kDa bound this site in UV cross-linking and Southwestern experiments. Levels of 4E regulatory factor activities correlated with c-Myc levels, eIF4E expression levels, and protein synthesis in differentiating U937 and HL60 cells, suggesting that these activities may function to regulate protein synthesis rates during differentiation. Since the eIF4E promoter lacked typical TATA and initiator elements, further studies of this novel initiator-homologous element should provide insights into mechanisms of transcription initiation and growth regulation.
Figures




References
-
- Cavanaugh A H, Hempel W M, Taylor L J, Rogalsky V, Todorov G, Rothblum L I. Activity of RNA polymerase I transcription factor UBF blocked by Rb gene product. Nature. 1995;374:177–180. - PubMed
-
- Chau C M, Evans M J, Scarpulla R C. Nuclear respiratory factor 1 activation sites in genes encoding the gamma-subunit of ATP synthase, eukaryotic initiation factor 2 alpha, and tyrosine aminotransferase. Specific interaction of purified NRF-1 with multiple target genes. J Biol Chem. 1992;267:6999–7006. - PubMed
-
- Chirgwin J M, Przybyla A E, MacDonald R J, Rutter W J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease activity. Biochemistry. 1979;18:5294–5299. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
