The locus control region is necessary for gene expression in the human beta-globin locus but not the maintenance of an open chromatin structure in erythroid cells
- PMID: 9742116
- PMCID: PMC109185
- DOI: 10.1128/MCB.18.10.5992
The locus control region is necessary for gene expression in the human beta-globin locus but not the maintenance of an open chromatin structure in erythroid cells
Abstract
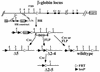
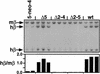
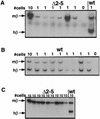
Studies in many systems have led to the model that the human beta-globin locus control region (LCR) regulates the transcription, chromatin structure, and replication properties of the beta-globin locus. However the precise mechanisms of this regulation are unknown. We have developed strategies to use homologous recombination in a tissue culture system to examine how the LCR regulates the locus in its natural chromosomal environment. Our results show that when the functional components of the LCR, as defined by transfection and transgenic studies, are deleted from the endogenous beta-globin locus in an erythroid background, transcription of all beta-globin genes is abolished in every cell. However, formation of the remaining hypersensitive site(s) of the LCR and the presence of a DNase I-sensitive structure of the beta-globin locus are not affected by the deletion. In contrast, deletion of 5'HS5 of the LCR, which has been suggested to serve as an insulator, has only a minor effect on beta-globin transcription and does not influence the chromatin structure of the locus. These results show that the LCR as currently defined is not necessary to keep the locus in an "open" conformation in erythroid cells and that even in an erythroid environment an open locus is not sufficient to permit transcription of the beta-like globin genes.
Figures



References
-
- Aladjem M I, Groudine M, Brody L L, Dieken E S, Fournier R E K, Wahl G M, Epner E M. Participation of the human β-globin locus control region in initiation of DNA replication. Science. 1995;270:815–819. - PubMed
-
- Amrolia P J, Ramamurthy L, Saluja D, Tanese N, Jane S M, Cunningham J M. The activation domain of the enhancer binding protein p45NF-E2 interacts with TAFII130 and mediates long-range activation of the α- and β-globin gene loci in an erythroid cell line. Proc Natl Acad Sci USA. 1997;94:10051–10056. - PMC - PubMed
-
- Bender, M. Unpublished data.
-
- Bender, M., A. Reik, J. Close, A. Telling, E. Epner, S. Fiering, R. Hardison, and M. Groudine. Description and targeted deletion of 5′HS 5 and 6 of the mouse β-globin locus control region. Blood, in press. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
