Biased suppression of hematopoiesis and multiple developmental defects in chimeric mice containing Shp-2 mutant cells
- PMID: 9742124
- PMCID: PMC109193
- DOI: 10.1128/MCB.18.10.6075
Biased suppression of hematopoiesis and multiple developmental defects in chimeric mice containing Shp-2 mutant cells
Abstract
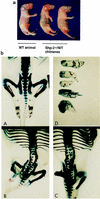
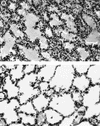
Shp-2 is a cytoplasmic tyrosine phosphatase that contains two Src homology 2 (SH2) domains at the N terminus. Biochemical data suggests that Shp-2 acts downstream of a variety of receptor and cytoplasmic tyrosine kinases. A targeted deletion mutation in the N-terminal SH2 (SH2-N) domain results in embryonic lethality of homozygous mutant mice at midgestation. In vitro embryonic stem (ES) cell differentiation assays suggest that Shp-2 might play an important role in hematopoiesis. By aggregating homozygous mutant (Shp-2(-/-)) ES cells and wild-type (WT) embryos, we created Shp-2(-/-)-WT chimeric animals. We report here an essential role of Shp-2 in the control of blood cell development. Despite the widespread contribution of mutant cells to various tissues, no Shp-2(-/-) progenitors for erythroid or myeloid cells were detected in the fetal liver and bone marrow of chimeric animals by using the in vitro CFU assay. Furthermore, hematopoiesis was defective in Shp-2(-/-) yolk sacs. In addition, the Shp-2 mutation caused multiple developmental defects in chimeric mice, characterized by short hind legs, aberrant limb features, split lumbar vertebrae, abnormal rib patterning, and pathological changes in the lungs, intestines, and skin. These results demonstrate a functional involvement of Shp-2 in the differentiation of multiple tissue-specific cells and in body organization. More importantly, the requirement for Shp-2 is more stringent in hematopoiesis than in other systems.
Figures





References
-
- Bash R O, Hall S, Timmons C F, Crist W M, Amylon M, Smith R G, Baer R. Does activation of the TAL1 gene occur in a majority of patients with T-cell acute lymphoblastic leukemia? A pediatric oncology group study. Blood. 1995;86:666–676. - PubMed
-
- Cooper, S., and H. E. Broxmeyer. 1996. Measurement of interleukin-3 and other hematopoietic growth factors, such as GM-CSF, G-CSF, M-CSF, erythropoietin and the other potent co-stimulating cytokines steel factor and Flt-3 ligand. Curr. Protocols Immunol. 18(Suppl.):6.4.1-6.4.12. - PubMed
-
- Deng C X, Wynshaw-Boris A, Shen M M, Daugherty C, Ornitz D M, Leder P. Murine FGFR-1 is required for early postimplantation growth and axial organization. Genes Dev. 1994;8:3045–3057. - PubMed
-
- de Vries C, Escobedo J A, Ueno H, Houck K, Ferrara N, Williams L T. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science. 1992;255:989–991. - PubMed
-
- Feng G S, Hui C C, Pawson T. SH2-containing phosphotyrosine phosphatase as a target of protein-tyrosine kinases. Science. 1993;259:1607–1611. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
