Amino acid residues that control pH modulation of transport-associated current in mammalian serotonin transporters
- PMID: 9742144
- PMCID: PMC6793029
- DOI: 10.1523/JNEUROSCI.18-19-07739.1998
Amino acid residues that control pH modulation of transport-associated current in mammalian serotonin transporters
Abstract
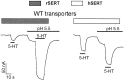
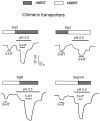
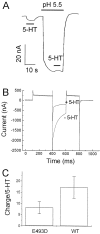
The rat and human serotonin transporters (rSERT and hSERT, respectively) were expressed in Xenopus oocytes and studied using site-directed mutagenesis, electrophysiological recordings, and [3H]5-HT uptake measurements. rSERT, but not hSERT, displayed increased transport-associated current at low pH. Chimeras and point mutations showed that, of the 52 nonidentical residues, a single residue at position 490 (threonine in rSERT and lysine in hSERT) governs this difference. Furthermore, potentiation required the glutamate residue at position 493. Cysteine substitution and alkylation experiments showed that residue 493 is extracellular. Cysteine at 493 increased, whereas aspartate decreased, the net charge movement per transported 5-HT molecule. The mutations at this region did not significantly affect other aspects of SERT function, including agonist-independent leakage current, voltage-dependent transient current, and H+ current. This region may therefore be part of an external gate required for rSERT function. The data and analyses show that, in the absence of detailed structural information, a gate-lumen-gate scheme is useful for interpreting results from mutations that alter functional properties of neurotransmitter transporters.
Figures





References
-
- Amara SG, Kuhar M. Neurotransmitter transporters: recent progress. Annu Rev Neurosci. 1993;16:73–93. - PubMed
-
- Barker EL, Kimmel HL, Blakely RD. Chimeric human and rat serotonin transporters reveal domains involved in recognition of transporter ligands. Mol Pharmacol. 1994;46:799–807. - PubMed
-
- Blakely RD, Berson HE, Fremeau RT, Caron MG, Jr., Peek MM, Prince HK, Bradley CC. Cloning and expression of a functional serotonin transporter from rat brain. Nature. 1991;354:66–70. - PubMed
-
- Buck KJ, Amara SG. Structural domains of catecholamine transporter chimeras involved in selective inhibition by antidepressants and psychomotor stimulants. Mol Pharmacol. 1995;48:1030–1037. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
