Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils
- PMID: 9742147
- PMCID: PMC6793017
- DOI: 10.1523/JNEUROSCI.18-19-07768.1998
Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils
Abstract
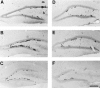
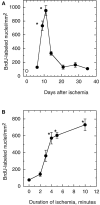
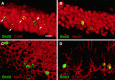
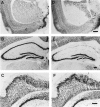
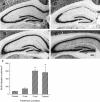
Neurogenesis in the dentate gyrus of adult rodents is regulated by NMDA receptors, adrenal steroids, environmental stimuli, and seizures. To determine whether ischemia affects neurogenesis, newly divided cells in the dentate gyrus were examined after transient global ischemia in adult gerbils. 5-Bromo-2'-deoxyuridine-5'-monophosphate (BrdU) immunohistochemistry demonstrated a 12-fold increase in cell birth in the dentate subgranular zone 1-2 weeks after 10 min bilateral common carotid artery occlusions. Two minutes of ischemia did not significantly increase BrdU incorporation. Confocal microscopy demonstrated that BrdU immunoreactive cells in the granule cell layer colocalized with neuron-specific markers for neuronal nuclear antigen, microtubule-associated protein-2, and calbindin D28k, indicating that the newly divided cells migrated from the subgranular zone into the granule cell layer and matured into neurons. Newborn cells with a neuronal phenotype were first seen 26 d after ischemia, survived for at least 7 months, were located only in the granule cell layer, and comprised approximately 60% of BrdU-labeled cells in the granule cell layer 6 weeks after ischemia. The increased neurogenesis was not attributable to entorhinal cortical lesions, because no cell loss was detected in this region. Ischemic preconditioning for 2 min, which protects CA1 neurons against subsequent ischemic damage, did not prevent increased neurogenesis in the granule cell layer after a subsequent severe ischemic challenge. Thus, ischemia-induced dentate neurogenesis is not attributable to CA1 neuronal loss. Enhanced neurogenesis in the dentate gyrus may be a compensatory adaptive response to ischemia-associated injury and could promote functional recovery after ischemic hippocampal injury.
Figures

References
-
- Altman J, Das G. Postnatal neurogenesis in the guinea-pig. Nature. 1967;214:1098–1101. - PubMed
-
- Bartesaghi R, Gessi T, Migliore M. Input–output relations in the entorhinal–hippocampal–entorhinal loop: entorhinal cortex and dentate gyrus. Hippocampus. 1995;5:440–451. - PubMed
-
- Bayer SA, Yackel JW, Puri PS. Neurons in the rat dentate gyrus granular layer substantially increase during juvenile and adult life. Science. 1982;216:890–892. - PubMed
-
- Bering R, Draguhn A, Diemer NH, Johansen FF. Ischemia changes the co-expression of somatostatin and neuropeptide Y in hippocampal interneurons. Exp Brain Res. 1997;115:423–429. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
