Regional and cellular patterns of reelin mRNA expression in the forebrain of the developing and adult mouse
- PMID: 9742148
- PMCID: PMC6792998
- DOI: 10.1523/JNEUROSCI.18-19-07779.1998
Regional and cellular patterns of reelin mRNA expression in the forebrain of the developing and adult mouse
Abstract
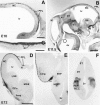
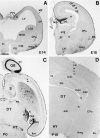
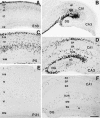
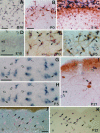
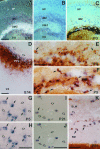
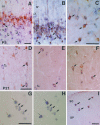
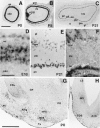
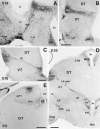
The reelin gene encodes an extracellular protein that is crucial for neuronal migration in laminated brain regions. To gain insights into the functions of Reelin, we performed high-resolution in situ hybridization analyses to determine the pattern of reelin expression in the developing forebrain of the mouse. We also performed double-labeling studies with several markers, including calcium-binding proteins, GAD65/67, and neuropeptides, to characterize the neuronal subsets that express reelin transcripts. reelin expression was detected at embryonic day 10 and later in the forebrain, with a distribution that is consistent with the prosomeric model of forebrain regionalization. In the diencephalon, expression was restricted to transverse and longitudinal domains that delineated boundaries between neuromeres. During embryogenesis, reelin was detected in the cerebral cortex in Cajal-Retzius cells but not in the GABAergic neurons of layer I. At prenatal stages, reelin was also expressed in the olfactory bulb, and striatum and in restricted nuclei in the ventral telencephalon, hypothalamus, thalamus, and pretectum. At postnatal stages, reelin transcripts gradually disappeared from Cajal-Retzius cells, at the same time as they appeared in subsets of GABAergic neurons distributed throughout neocortical and hippocampal layers. In other telencephalic and diencephalic regions, reelin expression decreased steadily during the postnatal period. In the adult, there was prominent expression in the olfactory bulb and cerebral cortex, where it was restricted to subsets of GABAergic interneurons that co-expressed calbindin, calretinin, neuropeptide Y, and somatostatin. This complex pattern of cellular and regional expression is consistent with Reelin having multiple roles in brain development and adult brain function.
Figures


References
-
- Ackerman SL, Kozak LP, Przyborski SA, Rund LA, Boyer BB, Knowles BB. The rostral cerebellar malformation gene encodes an UNC-5-like protein. Nature. 1997;386:838–842. - PubMed
-
- Angevine JB, Sidman RL. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature. 1961;192:766–768. - PubMed
-
- Anton ES, Marchioni MA, Lee K-F, Rakic P. Role of GGF/neuroregulin signaling in interactions between migrating neurons and radial glia in the developing cerebral cortex. Development. 1997;124:3501–3510. - PubMed
-
- Barth PG. Disorders of neuronal migration. Can J Neurol Sci. 1987;14:1–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
